Introduction
Regeneration of lost supporting tissues has always been considered the ideal objective of periodontal therapy. Periodontal regeneration refers to the restoration of supporting tissues of the teeth such as bone, cementum, and periodontal ligament to their original healthy levels before damage from periodontal bacteria has occurred. Several bone replacement grafts have been developed for use in periodontal therapy to support bone formation and defect fill. Calcium sulfate based bone grafting material has been used for 119 years with the first reported use of calcium sulfate from German physician Freidrich Trendlenberg in Bonn. Upon implantation in the body, it dissolves into calcium and sulfate ions. Calcium ions combine with phosphate ions from body fluids to form calcium phosphate. This serves as osseo conductive lattice of biologic apatite that stimulates bone in growth into the defect. This newly deposited material is mainly carbonated hydroxyl apatite which is similar to apatite naturally present in bone1.
Medical grade calcium sulfate hemi hydrate (CS) is completely synthetic, biocompatible, biodegradable and a highly osteo conductive material. It is the only bone graft that possesses haemostatic, angiogenic and barrier membrane properties. It is an effective pharmaceutical/growth factor delivery vehicle and can be used in combination with other bone graft materials2, 3.
Inspite of its superior properties, its fast degradation profile is a cause of concern in relatively large defects. CS undergoes degradation in 4 to 6 weeks.4 Nanocrystalline calcium sulfate was developed by a patented procedure to overcome this shortcoming §, 5. The bone graft stimulates bone regeneration in a controlled fashion.
The present study was undertaken to:
To evaluate clinically and radiographically the efficacy of two forms of calcium sulfate (CS and nCS) in the treatment of infrabony defects.
Materials And Methods
The present study was designed as a single- center randomized- controlled trial. The study was conducted at Department of Periodontics at D.A.V Centenary Dental College and Hospital, Yamuna Nagar, India, between April 2009 to February 2010. All clinical procedures were performed in accordance with the Declaration of Helsinki and the Good Clinical Practice Guidelines. Each patient provided written informed consent before participation and ethical clearance for the study was received from Ethical Committee, D.A.V Centenary Dental College and Hospital, Yamuna Nagar.
Patient and Defect Eligibility
Patients (a total of 30 sites in 12 patients)in the age group of 29- 62 years were included in the study in accordance to the following criteria: 1) moderate to advanced periodontitis along with radiographic evidence of infrabony defect; 2) no systemic diseases that contraindicated periodontal surgery; 3) no medications affecting periodontal status; 4) no pregnancy or lactation; and 5) presence of 1 deep (probing depth [PD] 6mm, radiographic depth 3mm) interproximal intra osseous periodontal defect. Third molars and teeth with degree III mobility, furcation involvement, or inadequate endodontic treatment or restoration were excluded. Smokers were also excluded from this study.
Experimental Protocol
Allocation. After subject selection (by RK), each intraosseous defect was randomly assigned to receive CS or nCS (by coin toss at the time of initiation of surgery). The clinical procedures differed only with respect to the graft material.
Presurgery procedures. Each patient underwent a full- mouth session of scaling and root planning using mechanical and hand instrumentation (by RK) and received personalized oral hygiene instructions. The surgical phase was delayed until the achievement of minimal residual inflammation and optimal soft tissue conditions at the defect site.
Regenerative material used in the study. CS (DentoGen, Orthogen, LLC, Springfield, NJ) is an FDA approved medical grade Calcium sulfate hemihydrate for bone regeneration in dentistry. CS is packaged in a cup in 1g quantities and comes with regular set and fast set liquid. Regular set liquid comprises of 0.9% sodium chloride solution while fast set liquid consists of 4% w/v potassium sulfate solution. For the present study regular set liquid was added to CS to form the putty.
nCS (NanoGen, Orthogen, LLC, Springfield, NJ) is particles of nanocrystalline calcium sulfate. This nanocrystalline bone graft material provides benefits of calcium sulfate with a slower degradation profile to allow for complete bone filling in large defects. Particle size of CS is 30-40µm. The pellets of nCS comes with a diameter of 425-1000µm. nCS retains the desirable properties of calcium sulfate and undergoes slower, controlled degradation than pure calcium sulfate.
Surgical Phase. All surgeries were performed by one periodontal surgeon (RK). The site of surgery was anesthetized using local anesthesia, 2% xylocaine with epinephrine 1:2, 00,000. Intra sulcular incisions with reflection of full thickness flaps were utilized to retain as much soft tissue as possible in order to obtain primary closure. Root and defect debridement were accomplished with hand instruments (Gracey Curettes). At the completion of surgical debridement, defects were filled with the assigned graft material. The graft material was emptied into a sterile dappen dish and prepared by adding slow set liquid drop by drop until it formed a paste like consistency. The paste was filled in the defect space and an effort was made to avoid contamination of the debrided root surface with saliva and blood until the graft material had been applied. The soft tissue flap was then repositioned at the original level and closed with interrupted direct loop sutures using 3-0 silk sutures. Care was taken to achieve a tension free primary closure of flap on suturing. Surgical site was protected by applying a periodontal dressing (Figure 1).
Post-Surgery Procedures. The patients were directed to abstain from mechanical oral hygiene procedures in the surgical area for 2 weeks. All the subjects were given both routine post-operative verbal and written instructions. A 0.12% chlorhexidine mouthrinse (10 ml twice a day) was used to support plaque control. They were prescribed medications which included a non-steroidal anti-inflammatory agent (Ibuprofen 400mg thrice a day for 3 days) for post-operative discomfort and an antibiotic Amoxicillin / Clavulanic acid (FlemiclavTM 625 mg twice a day for 3 days) to prevent infection. After 14 days, dressing, sutures and any plaque present in the area was removed. The recall appointments were scheduled at 3 months and 6 months post surgically for oral hygiene procedures and supra gingival plaque removal, soft tissue evaluation, radiographic evaluation and for recording of clinical parameters. Subgingival scaling was performed after completion of study at 6 months post- surgery.
Recordings. One examiner (NP) masked to the type of treatment received by the subjects, performed all pre- and post- treatment clinical and radiographic recordings using a UNC-15 graduated periodontal probe and a customized acrylic occlusal stents to provide reproducible testing points and insertion axes. A customized acrylic stent was fabricated on the study cast, for each patient, which was grooved in an occluso-apical direction to minimize variations in the direction of probing at subsequent recordings. All the measurements were made from a fixed reference point on the stent, which was done in accordance with the study by Orsini M et al (2008)6. The apical margin of the customized acrylic stent was used as the fixed reference point and the measurements were made at the proximal line angle of the tooth with bony defect. Only one site representing the same deepest point of the defect was included. Investigated Clinical Parameters included: 1) Plaque index (Silness and Loe (1964)) 7; 2) Gingival index (Loe and Silness (1963)) 8 (GI); 3) Probing Pocket depth (PD) and 4) Clinical attachment level (CAL).
Investigated Radiographic Parameters were: 1) Defect depth; 2) Amount of defect fill; 3) Percentage of defect fill; 4) Amount of defect resolution and 5) Percentage of defect resolution. The intraoral periapical radiographs of each defect site were taken pre-operatively and post-operatively using millimeter grid (X-ray Mesh). All radiographs were reviewed in a single reference centre by a masked evaluator. Defect depth was measured as the distance between the alveolar crest and the base of the defect. Defect fill was calculated as difference between initial and post-surgical radiographic bone level (distance from CEJ to bottom of intrabony defect) which then helped us determine the percentage of the defect fill. Defect resolution was calculated as the difference between initial and post-surgical defect depth, which helped us determine the percentage of defect resolution. All the parameters were evaluated at baseline, at 3 and 6 months post-surgically for all sites (Figure 1).
Statistical Analyses
A sample size of 15 (each group) was estimated to achieve 80 % power to detect a difference of 1.0 between null hypothesis and the alternative mean. A statistical software program (SPSS version 13.0) was used for data analysis. The statistical analysis was performed using the student t-test (paired and unpaired) for plaque index, gingival index, probing depth and clinical attachment level. Wilcoxon and Mann Whitney test was applied for defect fill, % defect fill, defect resolution and % defect resolution respectively. Mean values and standard deviations were calculated for each variable and examination interval.
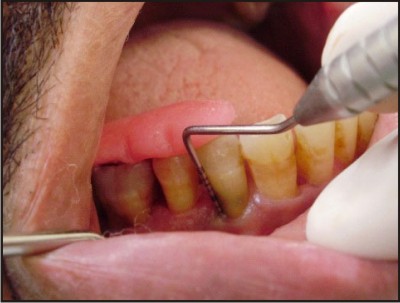 | A) Preoperative assessment done using customized acrylic stent and UNC-15 periodontal probe.
 |
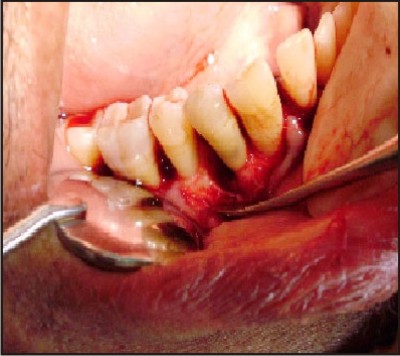 | B) Osseous defect after debridement in relation to mandibular right first premolar (distal).
 |
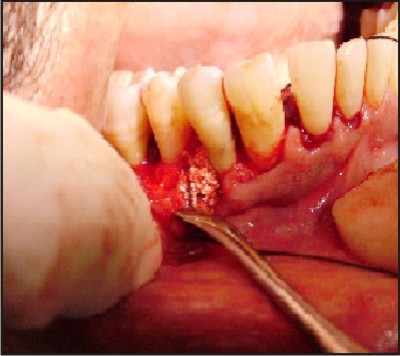 | C) CS placed and condensed into the osseous defect.
 |
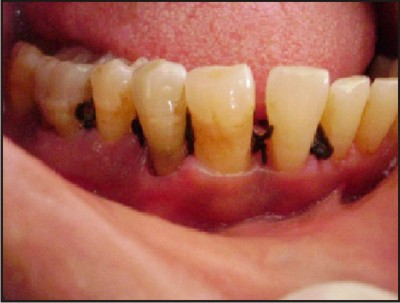 | D) Interrupted direct loop sutures given using 3-0 MersilkTM.
 |
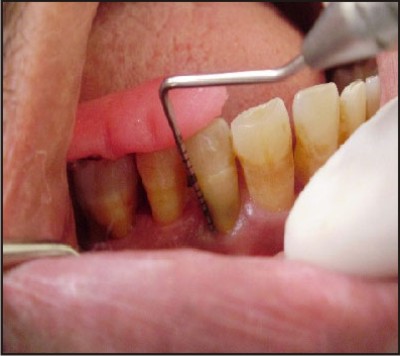 | E) Post operative assessment of clinical parameters at six months.
 |
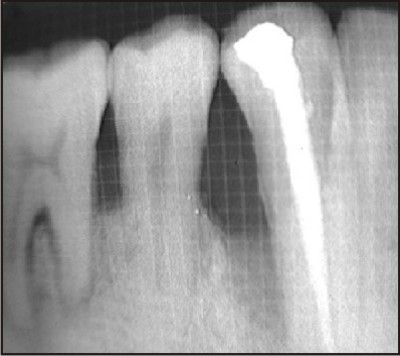 | F) Preoperative radiographic picture of the defect.
 |
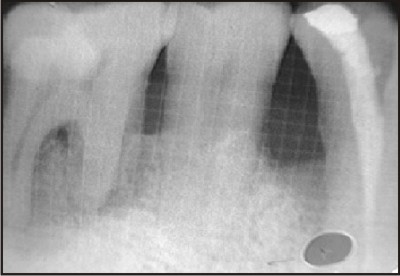 | G) Postoperative radiographic picture of the defect at three months.
 |
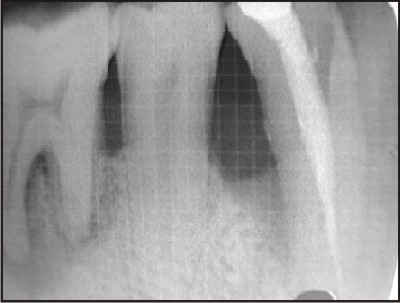 | H) Postoperative radiographic picture of the defect at six months.
 |
Results
Descriptive statistics
None of the patients were excluded from the analysis because of insufficient access or a defect morphology preventing root or defect instrumentation, or bone graft placement. Both the groups comprised of 15 sites each. All patients complied with the recall program until reevaluation. All defects were reevaluated at 3 and 6 months post- surgery.
Clinical recordings
Pre- and post- surgery clinical and radiographic recordings are reported in Table 1- 5.
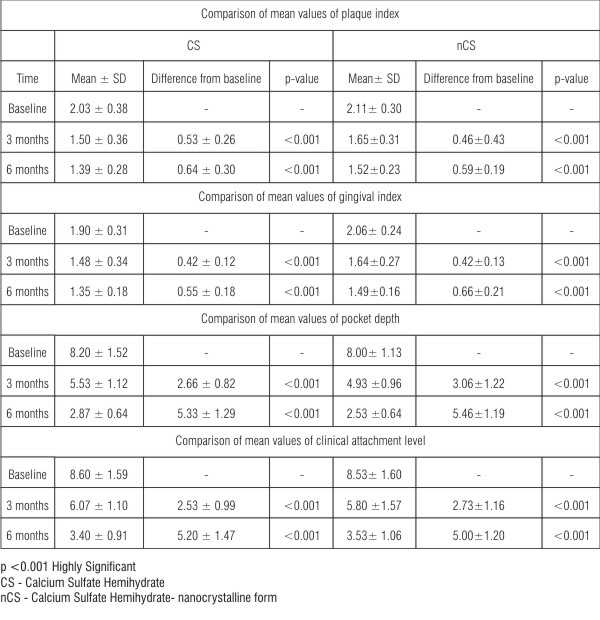 | Table 1: Comparison of mean values of plaque index, gingival index, pocket depth and clinical attachment level within the group (paired t-test)
 |
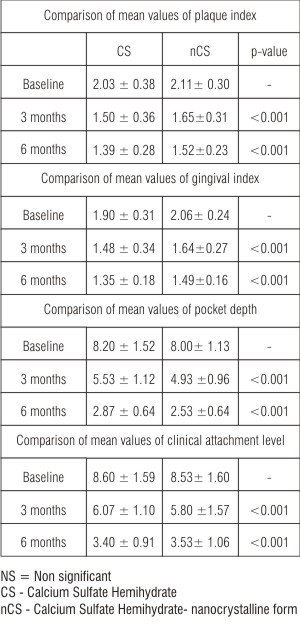 | Table 2: Comparison of mean values of plaque index, gingival index, pocket depth and clinical attachment level at baseline, 3 months and 6 months between CS and nCS groups (unpaired t-test)
 |
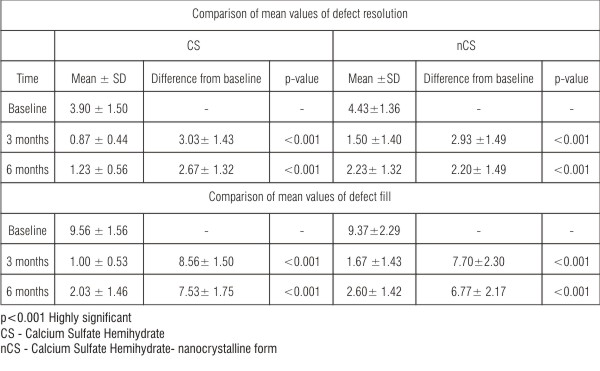 | Table 3: Comparison of mean values of defect resolution and defect fill (Wilcoxon test)
 |
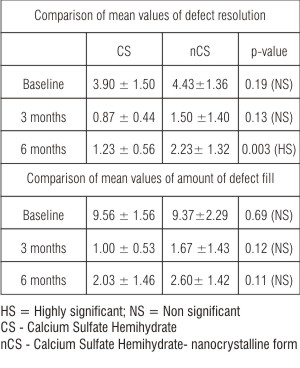 | Table 4: Comparison of mean values of defect resolution and defect fill at 3 months and 6 months between CS and nCS group (Mann Whitney test)
 |
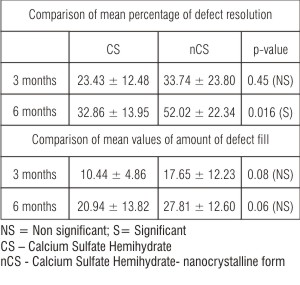 | Table 5: Comparison of mean percentage of defect resolution and defect fill at 3 months and 6 months between CS and nCS group (Mann Whitney test)
 |
The mean plaque index at baseline and after 3 months and 6 months post operatively for both the groups is given in Table 1. The mean difference of plaque index at 3 months and 6 months from baseline was 0.53 ± 0.26 and 0.64 ± 0.30 respectively for CS sites, and 0.46 ± 0.43 and 0.59 ± 0.19 respectively for nCS sites, both of which were highly significant (p<0.001) (Table 1) compared to baseline. However, there was no statistically significant difference in mean values of plaque index at baseline (p=0.54), at 3 months (p=0.24) and at 6 months (p=0.16) between the two groups (Table 2).
The mean gingival index at baseline and after 3 months and 6 months post operatively for both the groups is given in Table 1. The mean difference of gingival index at 3 months and 6 months from baseline was 0.42 ± 0.12 and 0.55 ± 0.18 respectively for CS sites and 0.42 ± 0.13 and 0.66 ± 0.21 respectively for nCS sites, both of which were highly significant (p<0.001) (Table 1) compared to baseline. However, there was no statistically significant difference in the mean values of gingival index at baseline (p=0.12), at 3 months (p=0.17) and at 6 months (p=0.42) between the two groups (Table 2).
The mean pocket depth at baseline and after 3 months and 6 months post operatively for both the groups is given in Table 1. The mean difference of pocket depth at 3 months and 6 months from baseline was 2.66 ± 0.82 and 5.33 ± 1.29 respectively for CS sites, and 3.06 ± 1.22 and 5.46 ± 1.19 respectively for nCS sites, both of which were highly significant (p<0.001), both at 3 and 6 months (Table 1) compared to baseline. However, there was no statistically significant difference in mean values of PD at baseline (p=0.69), at 3 months (p=0.13) and at 6 months (p=0.17), between the two groups (Table 2).
The mean CAL at baseline and after 3 months and 6 months post operatively for both the groups is given in Table 1. The mean difference of clinical attachment level at 3 and 6 months from baseline was 2.53 ± 0.99 and 5.20 ± 1.47 respectively for CS sites and 2.73 ± 1.16 and 5.00 ± 1.20 respectively for nCS sites both of which were highly significant (p<0.001) both at 3 and 6 months (Table 1) compared to baseline. However, there was no statistically significant difference in mean values of CAL at baseline (p=0.91), at 3 months (p=0.59) and at 6 months (p=0.71) between the two groups (Table 2).
Radiographic Recordings
The mean defect measurement at baseline was 9.56 ± 1.56 whereas the amount of defect fill at 3 and 6 months post operatively was 1.00 ± 0.53 and 2.03 ± 1.46 respectively for CS sites (Table 3). The mean defect measurement at baseline was 9.37 ± 2.29 whereas the amounts of defect fill at 3 and 6 months postoperatively were 1.67 ± 1.43 and 2.60 ± 1.42 respectively for nCS sites (Table 3). The mean difference of defect fill at 3 months and 6 months from baseline was 8.56 ± 1.50 and 7.53 ± 1.75 respectively for CS sites, and 7.70 ± 2.30 and 6.77 ± 2.17 respectively for nCS sites, both of which were highly significant (p<0.001) both at 3 and 6 months (Table 3) compared to baseline. There was statistically no significant difference in the mean values at baseline (p=0.69), at 3 months (p=0.12) and at 6 months (p=0.11) between the two groups (Table 4).
The mean percentage of defect fill from baseline to 3 months and 6 months post surgery was 10.44 ± 4.86 and 20.94 ± 13.82 respectively for CS sites and 17.65 ± 12.23 and 27.81 ± 12.60 respectively for nCS sites (Table 5). There was no statistically significant mean percentage of defect fill at 3 months (p=0.08) and 6 months (p=0.06) for CS and nCS sites (Table 5).
The mean defect depth at baseline was 3.90 ± 1.50 whereas the amount of defect resolution at 3 and 6 months post operatively was 0.87 ± 0.44 and 1.23 ± 0.56 respectively for CS sites. The mean defect depth at baseline was 4.43 ± 1.36 whereas the amount of defect resolution at 3 and 6 months postoperatively were 1.50 ± 1.40 and 2.23 ± 1.32 respectively for nCS sites (Table 3). There was statistically no significant difference in the mean values at baseline (p=0.19), and at 3 months (0.13) but statistically significant difference at 6 months (0.003) between the two groups (Table 4).
The mean difference of defect resolution at 3 months and 6 months from baseline was 3.03 ± 1.43 and 2.67 ± 1.32 respectively for CS sites and 2.93 ± 1.49 and 2.20 ± 1.49 respectively for nCS sites, both of which were highly significant (p<0.001) both at 3 and 6 months (Table 3) compared to baseline.
The mean percentage of defect resolution from baseline to 3 months and 6 months post surgery was 23.43 ± 12.48 and 32.86 ± 13.95 respectively for CS sites & 33.74 ± 23.80 and 52.02 ± 22.34 respectively for nCS sites. There was statistically significant difference in mean percentage of defect resolution at 6 months (p=0.16) and non significant difference at 3 months (p=0.45) for the two groups (Table 4, 5).
Discussion
Periodontal tissue regeneration of intrabony defects has been demonstrated by the use of different therapeutic modalities. Various bone grafts have been shown to be effective in treating periodontal disease. Trombelli et al (2002)9 and Reynolds et al (2003)10 in their systematic reviews summarized that bone replacement grafts and bone substitutes were significantly more effective than open flap debridement in improving attachment levels and in reducing probing depth. Among the different materials used in regenerative procedures, calcium sulfate is one of the first bone substitutes used in orthopedics and dentistry because of its beneficial properties6.
Calcium sulfate (CS) is a well-tolerated, biodegradable, osteoconductive bone graft substitute and is a reasonable alternative to autogenous bone graft. After being implanted into the bone defect, calcium sulfate undergoes degradation to calcium and sulfate ions. Calcium ions combine with phosphate ions from body fluids to form calcium phosphate, which provides an osteoconductive surface which stimulates the recruitment of osteoblasts and development of new bone in the defect. As calcium sulfate undergoes degradation in the body, there is a local decrease in pH. This pH drop results in demineralization of defect walls releasing bone growth factors which stimulate the formation and development of new bone.
While it has been used for more than a century, certain of its advantages have become apparent only in the past decade. It has been shown to serve as guided tissue regeneration barrier membrane on its own11. Pecora and colleagues (1997)12 concluded that it works as a barrier membrane by excluding growth of connective tissue and allowing bone regeneration. Recently, calcium sulfate was also observed to possess angiogenic properties. Strocchi et al (2002)13 demonstrated that more blood vessels grew into the defects filled with calcium sulfate than those filled with autograft. It can effectively be used as a drug delivery vehicle. Several drugs like Tobramycin (Beardmore et al in 2005)3, Simvastatin (Nyan et al in 2007)14 and Daptomycin (Webb et al in 2008)15 have been delivered locally through calcium sulfate.
However, calcium sulfate (CS) dissolves rapidly from the outer surface inwards at rate as high as 1 mm per week (it is usually completely degraded in 4 weeks).4 At times, its degradation outpaces the rate of new bone growth into the defect. To overcome this weakness, a nanocrystalline calcium sulfate (nCS) particles based bone graft was developed. Particles of nCS consist of densely packed grains of calcium sulfate in nanocrystalline form. nCS particles degrade in 12 to 14 weeks compared to standard CS, which degrade in 4 to 6 weeks. This material encourages robust bone growth and finds applications in various dental bone defects (intrabony defects, extraction sockets, sinus lifts, root perforations and apicoectomies) 4.
The present study was undertaken to evaluate two forms of calcium sulfate (CS and nCS).
This study showed statistically significant improvement in plaque and gingival indices at both follow up visits, when compared to the baseline levels (Table 1). There was no statistically significant difference in mean values of plaque index or gingival index at baseline, at 3 months and at 6 months between the two groups (Table 2). This was consistent with the studies conducted by Kim et al (1998)16, Stein JM et al (2009)17. The reduction of plaque and gingival scores could be attributed to the proper oral hygiene and patient compliance.
For the differences between the time points within each group, probing depth and clinical attachment level showed statistically significant reduction (Table 1). There was no statistically significant difference in mean values of pocket depth or CAL at baseline, at 3 months and at 6 months between the two groups (Table 2). These findings are in agreement with the results of Orsini M et al (2001)18, Couri CJ et al (2002)19 and Paolontonio M et al (2008)11. Levy et al (2002) stated that reduction in pocket depth by surgical means may be important in achieving sustained periodontal stability20.
The mean amount of defect fill from baseline to 3 months and 6 months was 1.00 mm and 2.03 mm respectively for CS sites which was statistically significant. The mean amount of defect fill from baseline to 3 and 6 months was 1.67 mm and 2.60 mm respectively for nCS sites which was also statistically significant (Table 3) compared to baseline. There was no statistically significant difference in the mean amount of defect fill at 3 months and 6 months between the two groups (Table 4). The mean percentage of defect fill at 3 months and 6 months in CS sites was 10.44% and 20.94% respectively and 17.65% and 27.81% for nCS sites respectively (Table 5).
The mean amount of defect resolution from baseline to 3 months and 6 months was 0.87 mm and 1.23 mm respectively for CS sites which was statistically significant. The mean amount of defect resolution from baseline to 3 and 6 months was 1.50 mm and 2.23 mm respectively for nCS sites which was also statistically significant (Table 3). There was no statistically significant difference in the mean amount of defect resolution at 3 months but was significant at 6 months between the two groups (Table 4). The mean percentage of defect resolution at 3 months and 6 months in CS sites was 23.43% and 32.86% and 33.74% and 52.02% for nCS sites which was statistically significant at 6 months (Table 5). These results are in consistency with the study done by Couri CJ et al (2002)19. This is consistent with the observation that calcium sulfate completely degrades in 4 to 5 weeks, whereas particles of nanocrystalline calcium sulfate show very little degradation at 4 weeks and takes up to 12 to 14 weeks for complete degradation.
The results of present study showed that calcium sulfate (CS and nCS) improves the healing outcomes vis-à-vis probing depth reduction and gain in clinical attachment level. Better biocompatibility, excellent handling properties, comparatively low cost, angiogenicity, barrier membrane and hemostatic properties are the explicit benefits of using calcium sulfate hemihydrates. nCS exhibited better defect fill and defect resolution than CS. No adverse effect or tissue response was observed with both the graft materials.
Defect fill and defect resolutions are the main outcomes that are usually reported by regenerative studies. 21 While defect fill takes into account only the changes at the base of the defect; defect resolution takes into account the changes in alveolar crest that may occur with regenerative therapy in addition to fill of the defect at the base. Thus, it may be interpreted that defect resolution is a better parameter. The most reliable outcome for assessing periodontal regeneration is histology. Due to ethical considerations, patient management limitations such as surgical re-entry and patient morbidity, no histological evaluation was made to establish proof of periodontal regeneration. Based on the histological evidence from human and animal trials, it is worth assuming that the clinical improvements following calcium sulfate treatment represents true periodontal regeneration22, 23, 24.
Keeping in view the small sample size of the present study it is recommended that further studies employing large study populations and longer evaluation time periods with frequent radiographic examination need to be conducted to analyze maximum potential of calcium sulfate hemihydrate in regenerative periodontics.
Conclusions
The present study was conducted to evaluate the efficacy of two forms of calcium sulfate hemihydrate for the treatment of infra bony defects. Clinical and radiographic evaluation was carried out at baseline, 3 months and 6 months for both the groups (CS and nCS).
The following conclusions were drawn from the present clinical study:
1. There was significant reduction in plaque index, gingival index and pocket depth in CS and nCS groups at 3 months and 6 months as compared to baseline.
2. There was no statistically difference in mean values of plaque index, gingival index and pocket depth at 3 months and 6 months between the two groups.
3. The gain in mean values of clinical attachment level at 3 months and 6 months were statistically significant as compared to baseline, however, the gain in clinical attachment level was statistically non significant after 3 months and 6 months when compared between the groups.
4. Both CS and nCS groups showed statistically significant amount of defect fill and defect resolution at 3 months and 6 months as compared to baseline.
5. A greater percentage of defect fill and resolution was noticed in nCS group.
6. Both graft materials can be considered valuable options in the treatment of infra bony periodontal defects. However, based on the results, nCS is more effective then CS.
7. The enhanced gain in defect fill and resolution of osseous defects in nCS could be attributed to its slow degradation profile that matches the rate of bone growth.
Acknowledgments
The bone graft materials used in the study (DentoGen and NanoGen) were provided by Orthogen LLC. The authors report no conflicts of interest related to this study.
References
1. Mamidwar S, Ricci JL, Harold A. Bone regeneration with calcium sulfate based bone grafts. Inside Dentistry. 2006; 2:1-7.
2. Orthogencorp.com [homepage on the internet]. New Jersey:Orthogen LLC;2008. Available from: http://www.orthogencorp.com/CS/ properties.html.
3. Beardmore AA, Brooks DE, Wenke JC, Thomas DB. Effectiveness of local antibiotic delivery with an osteoinductive and osteoconductive bone graft substitute. J Bone Joint Surg Am.2005; 87:107-12.
4. Ricci JL, Weiner MJ, Iorio DD, Mamidwar S, Alexander H. Evaluation of timed release calcium sulfate (CS-TR) bone graft substitutes. Microsc Microanal. 2005; 11:1256-7.
5. Orthogencorp.com [homepage on the internet]. New Jersey: Orthogen LLC; 2008. Available from: http://www.orthogencorp.com/rd/ CScr.html.
6. Orsini M, Orsini G, Benlloch D, Aranda JJ, Mariano S. Long term clinical results on the use of bone replacement grafts in the treatment of intrabony periodontal defects. Comparison of the use of autogenous bone graft plus calcium sulfate to autogenous bone graft covered with a bioabsorbable membrane. J Periodontol. 2008; 79:1630-7.
7. Loe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967; 38 (suppl): 610-6.
8. Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963; 21: 533-51.
9. Trombelli L, Heitz-Mayfield LJ, Needleman I, Moles D, Scabbia A. A systematic review of graft materials and biological agents for periodontal intraosseous defects. J Clin Periodontol. 2002; 29(Suppl. 3):117-35.
10. Reynolds MA, Aichelmann-Reidy ME, Branch-Mays GL, Gunsolley JC. The efficacy of bone replacement grafts in the treatment of periodontal osseous defects. A systematic review. Ann Periodontol. 2003; 8:227-65.
11. Paolantonio M, Perinetti G, Dolci M, Perfetti G, Tete S, Sammartino G et al. Surgical treatment of periodontal intrabony defects with calcium sulfate implant and barrier versus collagen barrier or open flap debridement alone: A 12 month randomized control clinical trial. J Periodontol. 2008; 79:1886-93.
12. Pecora G, Andreana S, Margarone JE, Covani U, Sottosanti JS. Bone regeneration with a calcium sulfate barrier. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997; 84:424-9.
13. Strocchi R, Orsini G, Iezzi G, Scarano A, Rubini C, Pecora G et al. Bone regeneration with calcium sulfate: Evidence for increased angiogenesis in rabbits. J Oral Implant. 2002; 28: 43-8.
14. Nyan M, Sato D, Oda M, Machida T, Kobayashi H, Nakamura T et al. Bone formation with the combination of simvastatin and calcium sulfate in critical sized rat calvarial defect. J Pharmacol Sci. 2007; 104:384-6.
15. Webb ND, McCanless JD, Courtney HS, Bungardner JD, Haggard WO. Daptomycin eluted from calcium sulfate appears effective against staphylococcus. Clin Orthop Relat Res. 2008; 466:1383-7.
16. Kim CK, Chai JK, Cho KS, Moon IS, Choi SH, Sottosanti JS et al. Periodontal repair in intrabony defects treated with a calcium sulfate implant and calcium sulfate barrier. J Periodontol. 1998; 69: 1317-24.
17. Stein JM, Fickl S, Yekta SS, Hoischen U, Ocklenburg C, Smeets R. Clinical evaluation of a biphasic calcium composite grafting material in the treatment of human periodontal intrabony defects: A 12- month randomized controlled clinical trial. J Periodontol. 2009; 80: 1774-82.
18. Orsini M, Orsini G, Benlloch D, Aranda JJ, Lazaro P, Sanz M et al. Comparison of calcium sulfate and autogenous bone graft to bioabsorbable membranes plus autogenous bone graft in the treatment of intrabony periodontal defects: A split mouth study. J Periodontol. 2001; 72: 296-302.
19. Couri CJ, Maze GI, Hinkson DW, Dawson DV. Medical grade calcium sulfate hemihydrate versus expanded polytetrafluoroethylene in the treatment of mandibular class II furcations. J Periodontol. 2002; 73: 1352-9.
20. Levy RM, Giannobile WV, Feres M, Haffajee AD, Smith C, Socransky SS. The effect of apically repositioned flap surgery on clinical parameters and the composition of the subgingival microbiota: A 12-Month Data. Int J Periodontics Restorative Dent. 2002 June; 22: 209-19.
21. Stavropoulos A, Karring T. Guided tissue regeneration combined with a deproteinized bovine bone mineral (Bio-Oss) in the treatment of intrabony periodontal defects: 6-year results from a randomized-controlled clinical trial. J Clin Periodontol. 2010; 37: 200-10.
22. Ruhaimi AKA. Effect of adding resorbable calcium sulphate to grafting materials on early bone regeneration in osseous defects in rabbits. Int J Oral Maxillofac Implants. 2000; 15:859-64.
23. Maeda ST, Bramante CM, Taga R, Garcia RB, Moraes IG, Bernadineli N. Evaluation of surgical cavities filled with three types of calcium sulfate. J Appl Oral Sci. 2007; 15:416-19.
24. Silveira RL, Machado RA, Silveira CRS, Oliveira RB. Bone repair process in calvarial defects using bioactive glass and calcium sulfate barrier. Acta Cirurgica Brasileira. 2008; 23:322-8. |