Introduction:
Oral health is an important aspect of overall health status of an individual. Teeth and their supporting (periodontal) structures are of importance to oral health. Periodontitis is an inflammatory disease of the peridontium, which affects the supporting tissues of the teeth [1]. The human oral cavity is colonized by a number of microbes, especially bacteria. Often, bacteria and host cells form a commensal relationship which is beneficial for both, but under certain conditions (increased mass or pathogenicity, reduced host immune response), disease may occur. Specific bacteria induce the release of cytokines which increase the number of defense cells and their activation [2, 3]. Reactive oxygen species (ROS) such as superoxide anion, hydroxyl radical, nitrous oxide and hydrogen peroxides are produced via the bacteria- host mediated pathway, stimulating polymorphonuclear leucocytes (PMNL) to produce superoxide radicals via “respiratory burst”. This result in increased ROS concentration leading to oxidative damage to periodontal tissues with an impaired circulating antioxidant: oxidant balance [4, 5].
All mammalian cells contain antioxidants (AOs) that prevent or limit oxidative tissue injury caused by ROS [6]. AOs may be enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GR), high molecular weight proteins such as albumin, ceruloplasmin, transferrin, low molecular weight water soluble antioxidants such as ascorbic acid, uric acid etc and low molecular weight water insoluble antioxidants such as tochopherol, carotenoids, bilirubin etc. AOs overall, act at different levels of action such as prevention, interception and repair damage caused by ROS. In a normal healthy human body the generation of free radicals or pro-oxidants is effectively kept in check at various levels by antioxidant defense mechanism. However, when it gets exposed to adverse physico-chemical, environmental or pathological agents, this delicately maintained balance (antioxidant: oxidant) shifts in favor of pro-oxidants, resulting in oxidative stress [7, 8 and 9].
The present study tries to evaluate and compare some biochemical oxidative stress markers in peripheral blood of patients with chronic periodontitis and healthy controls.
Materials and Methods:
Study Group:
A total of 60 subjects: 30 patients with chronic periodontitis (19 males and 11 females, with a mean age of 41.6 ± 6.4) and 30 healthy controls (19 males and 11 females, with a mean age of 39.6 ± 8.4) were referred to the Department of Dentistry, Grant Medical College, Mumbai. The patients in the study group were otherwise healthy, with no history of major illness and consumption of antioxidants, antibiotics, anti inflammatory or any other drugs for at least six months. Subjects having past illness and undergoing any treatment, smokers, alcoholics, pregnant and lactating women were excluded from the study. All the individuals had a minimum of 20 teeth present. The individuals in the control group were from the same geographical region with good oral health.
The study was undertaken as per the approval of the Institutional Ethics Committee of Grant Medical College and Sir J. J. Group of Hospitals, Mumbai. A written informed consent was obtained from all the subjects enrolled for the study.
Clinical measurements:
The periodontal status of all individuals was evaluated by measurement of gingival index (GI) as developed by Loe H and Silness J , plaque index (PI) as described by Silness P and Loe H, papillary bleeding index (PBI) developed by Muhlemann HR [10] and clinical attachment loss (CAL). CAL was measured on six sites of each tooth (mesial, median and distal points at buccal and palatal aspects). The individual scores were compared on a scale for characterization of periodontitis as slight, moderate or severe [11]. All clinical measurements were evaluated by a single investigator.
Sample Collection:
A total of 4 ml venous blood was collected in disposable syringe. Of this, 1 ml heparinised blood was used for analysis of RBC-SOD, blood GPx and plasma TAOC. The remaining 3ml of blood was allowed to stand at room temperature for 30 min and later centrifuged to obtain serum, which was used for the analysis of Vitamin C, MDA and CRP.
Biochemical studies:
The plasma TAOC was measured by the Ferric Reducing Ability of Plasma (FRAP) assay [12]. The reaction measures antioxidant reduction of Fe3+TPTZ (tripyridyl triazine) to Fe2+TPTZ and the change in absorbance was measured at 593 nm.
The RBC-SOD was measured using the kit method, obtained from Randox Laboratories (U. K). 1 unit of enzyme activity was defined to cause 50% inhibition of rate of reduction of 2, 4 -idophenyl 3-4 nitrophenol 5-phenyl tetrazolium chloride (INT) under assay condition. Change in absorbance was recorded at 505 nm [13].
Blood GPx was also measured using kit method, obtained from Randox Laboratories (U. K). GPx catalyses oxidation of glutathione by cumene hydroperoxide. The oxidized glutathione is reduced by glutathione reductase and the decrease in absorbance was measured at 340 nm [14].
The serum vitamin C content was measured using the dinitro phenyl hydrazine (DNPH) method. In strong acidic medium, oxidized ascorbic acid reacts with 2, 4 DNPH to form a red coloured complex which was measured at 500 nm [15].
The serum MDA was estimated using thiobarbituric acid method. A pink colored complex is obtained whose absorbance was measured at 530 nm [16].
The serum CRP was measured by the latex turbidity method using the kit obtained from Spinreact (Spain). The change in absorbance was measured at 540 nm [17].
Statistical Analysis:
The measured values for the clinical parameters and biochemical markers were subjected to statistical analysis. The values were expressed as mean± SD. The p values for mean ± SD were obtained by Mann-Whitney U test. p value less than 0.05 is considered to be statistically significant.
Observations and Results:
Clinical measurements:
The values of clinical measurements are listed in Table 1. All clinical parameter scores (mean ± SD) were significantly higher (p<0.001) in chronic periodontitis patients compared to healthy controls.
Biochemical Studies:
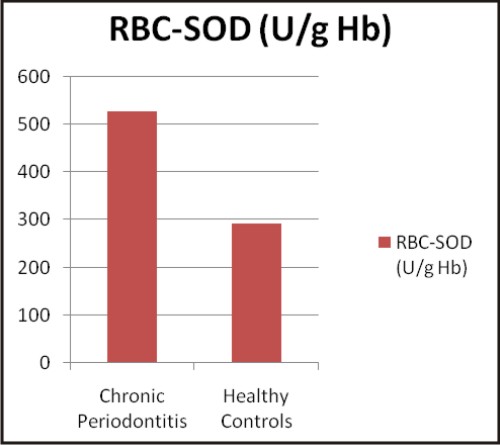
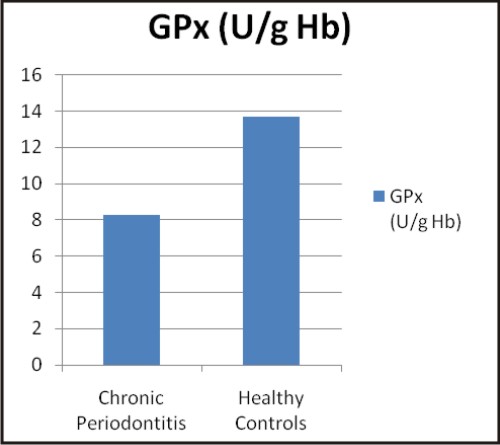
The values of biochemical parameters evaluated in the present study are shown in Table 2. The values are expressed as mean ± SD and were found to be significant (p<0.001) in chronic periodontitis patients when compared to healthy controls.
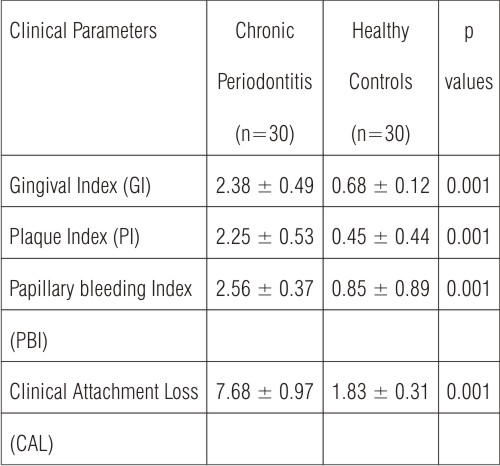 | Table 1: Clinical parameters: p values for mean ± SD obtained by Mann-Whitney U test
 |
 | Table 2: Biochemical parameters: p values for mean ± SD obtained by Mann-Whitney U test
 |
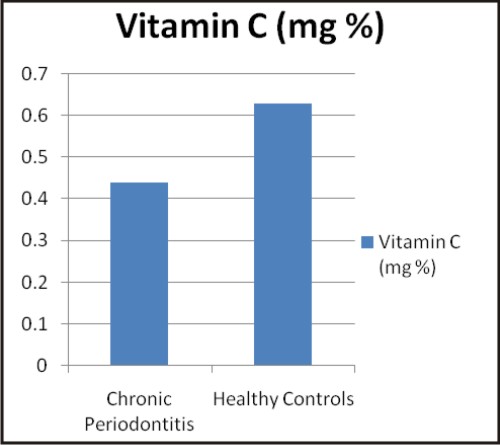
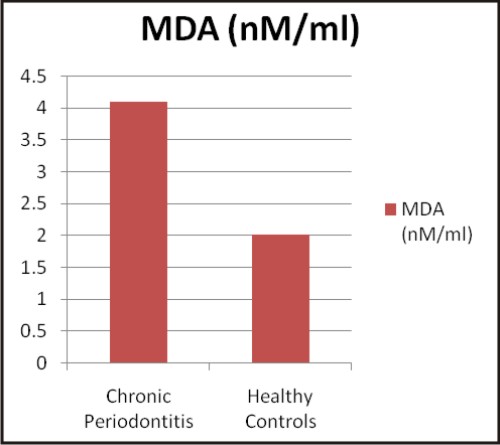
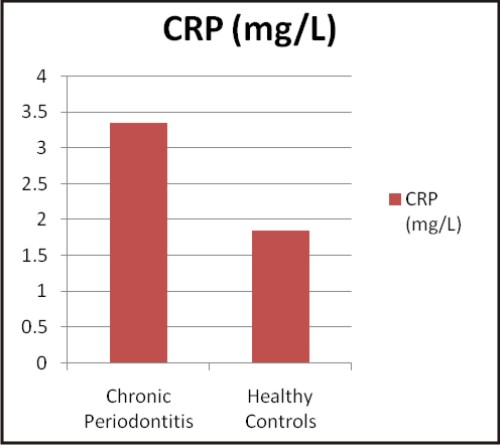
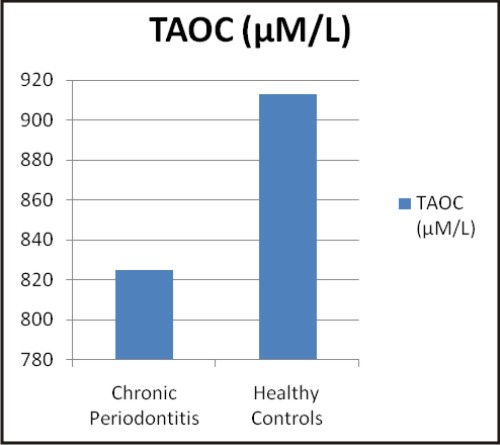 | Graphs: Comparison between the different biochemical oxidative stress markers in chronic periodontitis and healthy controls (a) TAOC (µM/L), (b) RBC-SOD (U/g Hb), (c) GPx (U/g Hb), (d) Vitamin C (mg %) (e) MDA (nM/ml) and (f) CRP (mg/L) (A)
 |
 | (B)
 |
 | (C)
 |
 | (D)
 |
 | (E)
 |
 | (F)
 |
Discussion:
Periodontal disease has long been recognized as a public health problem. Periodontitis is caused by multi factorial process triggered by infection of peridontium with Gram negative bacteria. The response of the host to most periodontal infection is chronic inflammation. This response has both local and systemic inflammatory manifestations [18]. These inflammatory manifestations may lead to oxidative stress. The present study has focused on the relationship between periodontitis and biochemical oxidative stress markers.
Total antioxidant capacity (TAOC) reflects full spectrum of antioxidant activity against various reactive oxygen and nitrogen radicals. The total antioxidant assay measures predominantly the low molecular weight chain breaking antioxidants, such as urate, ascorbate, bilirubin etc. Generally, low total antioxidant capacity indicates oxidative stress or increased susceptibility to oxidative damage [19].
Oxidative stress is implicated in the pathogenesis of periodontitis. Various studies have associated periodontitis with TAOC. Capple IL (2007) [20], observed lower GCF TAOC levels in chronic periodontitis compared to healthy controls. However, plasma TAOC has not shown significant differences in chronic periodontitis and healthy controls. The present study has observed lowered plasma TAOC in patients with chronic periodontitis as compared to healthy controls. Similar to the present findings, various studies have observed compromised TAOC in chronic periodontitis compared to healthy controls. Konopka T et al (2007) [21] and Canakci V. et al (2007) [22] have observed lower TAOC in saliva, gingival blood and peripheral blood of subjects with chronic periodontitis as compared to the healthy control group. Similarly Baltacioqlu E et al (2006) [23], Akalin FA (2009) [24], D' Aiuto F et al (2010) [25] and Abou Sulaiman et al (2010) [26] have associated reduced antioxidant capacity with periodontitis. The significantly decreased Total Antioxidant Status (TAS), in the peripheral blood in chronic periodontitis may be one of the pathogenic mechanisms underlying the link between periodontal disease and several systemic diseases [21].
Antioxidant enzymes, superoxide dismutase (SOD) and glutathione peroxidase (GPx) provides protection within the cell against ROS. Three isoenzymes of SOD are known in humans, Cu-Zn- SOD found in cytoplasm and nucleus, mitochondrial Mn-SOD and extracellular Cu-Zn-SOD. Two forms of GPx are known, classical cellular GPx and extracellular GPx (eGPx), of which eGPx serves an important antioxidant role in many extracellular surfaces and spaces [27]. The function of SOD is to remove damaging ROS from the cellular environment by catalyzing dismutation of two super oxide radicals to hydrogen peroxide (H2O2). GPx reduces H2O2 by the oxidation of reduced glutathione. In contrast to our findings, Baltacioqlu E et al 2006 [23], Canakci V et al 2007 [22] and Akalin F A et al 2008 [28] have observed significantly lower SOD activities in chronic periodontitis patients.
The human periodontal ligament has shown to possess the enzyme SOD which offers biological protection against ROS. Bacterial lipopolysaccharides also stimulates superoxide release from gingival fibroblast, suggesting that the induction of SOD may represent an important defense mechanism of the fibroblasts during inflammation [29]. In the present study increased RBC-SOD activity in chronic periodontitis supports the above findings as part of the systemic response. Various studies conducted by Panjamurthy K et al 2005 [30], Wei D et al 2010 [29] and Tonque M O et al 2011 [31] have observed higher SOD activity in chronic periodontitis group than in controls which is in accordance with our study.
The present study has obtained lower GPx activity in whole blood of chronic periodontitis group than in controls. The study conducted by Tsai CC et al 2005 [32] has shown no significant change in salivary GPx in chronic periodontitis and healthy controls. Patel S P et al 2009 [4] have observed direct proportionality in GPx activity in GCF with the severity of periodontal disease. However, consistent with our findings Canakci V et al 2007 [22], Canakci F C et al 2009 [27] and Tonque M O et al 2011 [31] have shown lower GPx activity in chronic periodontitis compared to healthy controls.
Vitamin C (Ascorbic acid) is a low molecular weight, water soluble antioxidant. It has protective effect on maintaining tissue homeostasis by playing important role in collagen synthesis and therefore helps in maintenance of structural integrity of the connective tissue. Vitamin C also has a beneficial role as radical scavenger [33]. Vitamin C, through its antioxidant action, neutralizes oxidative stress, and in doing so may be depleted in plasma. It is therefore quite possible that periodontitis causes lower plasma vitamin C through this mechanism [34]. In our study serum vitamin C level was lower in chronic periodontitis as compared to healthy controls which was also found by Staudte H et al 2005 [35],Anwar TM 2007 [34], Chapple IL et al 2007 [33], Thomas B et al 2010 [36] and Van der Velde U 2011 [37].
Lipid peroxidation (LPO) has been implicated in the pathogenesis of several pathological disorders including periodontal disease. ROS, can attack polyunsaturated fatty acids and induce formation of LPO products such as MDA. It is a stable end product of peroxidation of lipids by ROS. MDA is one of the most frequently used indicator of lipid peroxidation and may be a potential biomarker indicating oxidative stress [38]. Our finding indicates higher serum MDA level in chronic periodontitis as compared to the healthy controls. Many researchers like Borges I Jr et al 2007 [39], Khalili J 2008 [40], Canakci FC 2009 [27], Wei D et al 2010 [29] and Tonque MO et al 2011 [31] have also observed higher MDA levels in chronic periodontitis.
C - Reactive protein (CRP) is synthesized by the liver and circulates in the blood, the levels of which rise in response to inflammation i.e. CRP is an acute phase protein, which is used as an inflammatory marker. CRP during its role in inflammatory process binds to the surface of pathogens and opsonises them for the process of phagocytosis. CRP can also activate the classic complement cascade by binding to 'q' factor of complement factor 1 (C1q). It has been demonstrated that CRP levels are higher in periodontitis patients than in healthy subjects [41]. Studies conducted by Nadeem M et al 2009 [42], Gani DK et al 2009 [43], Thakare KS 2010 [44], and Masi S et al 2011 [45] have observed elevated CRP levels in chronic periodontitis as compared to the control group, which supports our findings.
The balance between ROS and antioxidant defense mechanism is likely to be important in periodontal pathogenesis. As observed in our study, patients with chronic periodontitis have shown higher inflammatory and oxidative stress markers, and lowered antioxidant defense. Further studies based on large sample size are needed to establish these findings and also to reveal the mechanism of pathogenesis of periodontitis at a molecular level.
Conclusion:
The study can be concluded on the note that, patients with chronic periodontitis show higher oxidative stress which is evident from the levels of biochemical oxidative stress markers. The imbalance between oxidant and antioxidant reflects higher ROS activity which leads to periodontal inflammation and its pathogenesis. Our study helps to add to the existing knowledge of periodontitis as an inflammatory disorder and its association with systemic biochemical oxidative stress markers, which may be of help in clinical management of the disease.
References:
1. Kinane DF. Causation and pathogenesis of periodontal disease. Periodontol 2000 2001;25:8-20
2. Marc Q, Wim T, Susan KH, Michael GN. Microbiology of periodontal disease. Carranza's Clinical Periodontology 10th ed. St Louis, Saunders/Elsivier 2006;134-149
3. Maria ER, Philip MP. Host modulation. Carranza's Clinical Periodontology 10th ed. St Louis, Saunders/Elsivier 2006;275-82
4. Patel SP , Pradeep AR, Chowdhry S. Cervicular fluid levels of plasma glutathione peroxidase (eGPx) in periodontal health and disease. Arch Oral Biol 2009 Jun;54(6):543-48
5. Akalin FA, Toklu E, Renda N. Analysis of SOD activity levels in gingival and GCF in patients with chronic periodontitis and periodontally healthy controls. J Clin Periodontol 2005;32(3):238-43
6. Chapple IL, Mathews JB. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol 2000 2007;43:160-232
7. Djordjevic VB. Free radicals in cell biology. Int Rev Cytol 2004;237:57-89
8. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007;39:44-84
9. Maurya DK, Kumar SS, Devasagayam TPA. Free radicals, cancer and cardiovascular disease. Conference proceedings-National conference on oxidation stress and its complications in human health 2011 Jan;7-19
10. Soben P. Essentials of preventive and community dentistry. Indices in dental epidemiology. 2nd ed. Arya Med Pubc2003;127-240
11. John MN. Classification of disease and condition affecting the periodontium. Carranza's Clinical Periodontology 10th ed. St Louis, Saunders/Elsivier 2006;100-109
12. Iris FF, Benzie, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power" - the FRAP assay. Analytical Biochemistry 1996;239(1):70-76
13. Woolliams JA, Wiener G, Anderson PH, McMurray CH. Research in veterinary science 1983; 34:253-56
14. Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 1967;70:158-169
15. Harold V. Carbohydrates. Practical Clinical Biochemistry 5th ed 2005;173-76
16. Kei S. Serum lipid peroxide in cerbrovascular disorders determined by a new colorimetric method. Clinica Chemica Acta 1978;90:37-43
17. Lars O H. Current opinion in infectious diseases 1997;10:196-201
18. Dave S, Balista EL Jr, Thomas E, van Dyke. Cardiovascular disease and periodontal disease: commonality and causation. Compendium 2004 July;25(7)Supp1:26-37
19. Young IS. Measurement of total antioxidant capacity. J Clin Pathol 2001;54:339
20. Chapple IL, Brock GR, Milward MR. Compromised GCF TAOC in periodontitis: cause or effect. J Clin Periodontol 2007 Feb;34(2):103-10
21. Konopka T, Krol K, Kopec W, Gerber H. Total antioxidant status and 8 - hydroxyl-2-deoxyguanosine levels in gingival and peripheral blood of periodontitis patients. Arch Immunol Ther Exp 2007 Nov-Dec;55(6):417-22
22. Canakci V, Yildrim A, Canakci CF, Eltas A, Cicek Y, Canakci H. Total antioxidant capacity and antioxidant enzymes in serum, saliva and gingival cervicular fluid of preeclamptic women with and without periodontal disease. J Periodontol 2007 Aug;78(8):1602-11
23. Baltacioqlu E, Aklain FA, Alver A, Balafan F, Unsal M, Karabulut E. Total antioxidant capacity and SOD activity levels in serum and GCF fluid in post menopausal women with chronic periodontitis. J Clin Periodontol 2006 Jun;33(6):385-92
24. Akalin FA, Baltacioqlu E, Alver A, Karabulut E. Total antioxidant capacity and super oxide dismutase activity levels in serum and gingival cervicular fluid in pregnant women with periodontitis. J Periodontol 2009 Mar;80(3):457-67
25. D' Aiuto F, Nibali L, Parker M, Patel K, Suran T, Donos N. Oxidative stress, systemic inflammation and sever periodontitis. J Dent Res 2010 Nov;89(11):1241-46
26. Abou Sulaiman AE, Shehadeh RM. Assessment of total antioxidant capacity and use of vitamin C in the treatment of non smokers with chronic periodontitis. J Periodontol 2010 Nov;81(11):1547-54
27. Canakci CF, Geek Y, Yildirim A, Sezer U, Canakci V. Increased levels 8 - hydroxyl-2-deoxyguanosine and MDA and its relationship with antioxidant enzymes in saliva of periodontitis patients. Eur J Dent 2009 Apr;3(2):100-106
28. Akalin FA, Isiksal E, Baltacioqlu E, Renda N, Karafulut E. SOD activity in gingival in type 2 diabetes mellitus patients with chronic periodontitis. Arch Oral Biol 2008 Jan;53(1):44-52
29. Wei D, Zhang XL, Wang YZ, Yang CX, Chen G. Lipid peroxidation level, total oxidant status and super oxide dismutase in serum, saliva and gingival cervicular fluid in chronic periodontitis patients before and after periodontal therapy. Aust Dent J 2010 Mar;55(1):70-8
30. Parjamurthy K, Manoharan S, Ramchandran CR. Lipid peroxidation and AO status in patients with periodontitis. Cell Mol Biol Lett 2005;10(2):255-64
31. Tonque MO, Ozturk O, Sutcu R, Cryhan BM, Kilinc G, Sonmez Y et al. The impact of smoking status on antioxidant enzyme activity and MDA level in chronic periodontitis. J Periodontol 2011 Jan; (Epub ahead of print)
32. Tsai CC, Chen HS, Chen SL, Ho YP, Ho KY, Wu YM et al. Lipid peroxidation : a possible role in the induction and progression of chronic periodontitis. J Periodontol Res 2005 Oct;40(5):378-84
33. Chapple LC, Milward MR, Dietrich T. The prevalence of inflammatory periodontitis is negatively associated with serum antioxidant concentrations. J Nutr 2007 Mar;137:657-64
34. Anwar TM. Plasma Vitamin C is Inversely Associated with Periodontitis. J Evid Base Dent Pract 2008;8:103-104
35. Staudte H, Siqusch BW, Glockmann E. Grapefruit consumption improves vitamin C status in periodontitis patients. Br Dent J 2005 Aug;199(4):213-17
36. Thomas B, Kumari S, Ramitha K, Ashwini Kumari MB. Comparative evaluation of micronutrients in the serum of diabetes mellitus patients and healthy individuals with periodontitis. J Indian Soc of Periodontal 2010 Jan;14(1):46-9
37. Van der U, Kuzmanova D, Chapple IL. Micro nutritional approaches to periodontal therapy. J Clin Periodontal 2011 March;38Supp11:142-58
38. Nielsen F, Mikkelson BB, Nicholson JB, Anderson HR, Grandjean P. Plasma MDA as biomarker and oxidative stress: reference interval and effects of life style pattern. Clinical chemistry 1997;43:1209-14
39. Borges I Jr, Moeira EA, Filho DW, Oliveira TB, da Silva MB, Frode TS. Proinflammatory and oxidative stress markers in patients with periodontal disease. Mediators Inflamm 2007; 2007:45794
40. Khalili J, Bilokytska HF, SalivarKhalili J, Bilokytska HF. Salivary MDA levels in clinically healthy and periodontal diseased individuals. Oral Dis 2008 Nov; 14(8):754-60
41. Gupta SC, Jindal V, Ranbika T. CRP and its role in periodontitis. Indian J of Dental Sciences 2011;3(1):31-32
42. Naddem M, Stephen L, Schibert C, Dacids MR. Association between periodontitis and systemic infection in patients with ESRD. SADJ 2009 Nov;64(10):470-73
43. Gani DK, Lakshmi D, Krishnan R, Emmadi P. Evaluation of CRP and interleukins - 6 in the peripheral blood of patients with chronic periodontitis. J Indian Soc Periodontol 2009 May; 13(2):69-74
44. Thakare KS, Deo V, Bhongade ML. Evaluation of CRP serum levels in periodontitis patients with or without atherosclerosis. Indian J Dent Res 2010 Jul-Sep;21(3)326:29
45. Masi S, Salpea KD, Li K, Parkar M, Nibali L, Donos N et al. Oxidative stress, chronic inflammation and telomere length in patients with periodontitis. Free Radic Biol Med 2011 Mar; 50(6):730-35
|