Introduction:
CHX is a bisbiguanide and has been shown to possess antibacterial properties owing to its broad antimicrobial spectrum. It has been used extensively to treat endodontic infections [1] and periodontal diseases [2]. Also its role as a caries preventive agent has been evaluated and proven [3]
Of late, studies have been undertaken to analyze the role of CHX in stabilizing the organic matrix of resin-dentin bond which have shown that CHX has a beneficial role in the preservation of resin-dentin bond [4,5]. This property of CHX probably relates to its anti MMP-2, MMP -8 & MMP 9 activity. The clinical efficiency of CHX also relates to its property of substantivity which ensures its release and availability at the site delivered for a considerable period of time [6]. It is this property of CHX that makes the dentin to form hybrid layers that are more stable once treated with CHX. [7]
Considering the importance of this property of CHX in the field of restorative dentistry, it seems necessary to evaluate the concentration of CHX which can be clinically made use of in this field. The purpose of this study was to evaluate the substantivity of CHX in two different concentrations so as to make a clinical recommendation.
Materials And Method:
Fifty extracted non carious mandibular third molars were collected after the informed consent of the patients and were stored in 0.9% sodium chloride containing 0.02% sodium azide at 4 degree Celsius for 20 days. After the removal of debris, the roots were removed, and pulp tissue was also removed. Enamel and Cementum was removed using diamond points and water as a coolant. Diamond saw was used to make dentin discs from the remaining tooth portion. The disc size was kept approx. to be 5mm in diameter and 2 mm in thickness (Fig 1). The discs were dried and then immersed in distilled water at 37 degree Celsius for one week and their wet mass was recorded. The part of the disc that was initially in contact with the pulp chamber was covered with two layers of nail varnish and dried. The dry mass was also recorded. All the discs were partially demineralised using liquid 37% phosphoric acid for 15 seconds and then washing it off with distilled water for 60 seconds.
 | Fig 1 : Dentin Disc
 |
The discs were then divided into two groups. Group A discs were
treated with 10 microlitre of 0.2% CHX (Fig 2) which was applied for 20 seconds (Fig 3) while Group B discs were treated with 10 microlitre of 2% CHX (Fig 4).
 | Fig 2 : 10 Microlitre Of Chlorhexidine Solution
 |
 | Fig 3 : 0.2 % Chlorhexidine Solution Being Applied On Dentin Disc
 |
 | Fig 4 : 2 % Chlorhexidine Solution Being Applied On Dentin Disc
 |
The discs were then transferred to 2 ml plastic centrifuge tubes having 1 ml of PBS and incubated at 37 degree Celsius [8]. One ml of PBS solution was taken from each tube and spectrophotometric analysis was done at 260 nm after 1 hr, 24 hr and one week of incubation to estimate the concentration of CHX (in percentage) in the solution.
Observations And Results:
UV absorbance was recorded for all specimens (in terms of percentage of CHX remaining bound) and is shown in Table 1. Higher substantivity was observed for specimens in Group A as compared to Group B (p<0.05, statistically significant).
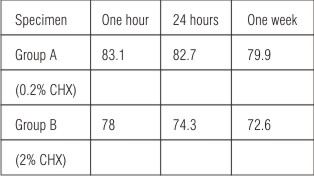 | Table 1: Percentage of chlorhexidine remaining bound
 |
Discussion:
Use of CHX for its antibacterial action in the field of endodontics as well as periodontics is world wide accepted. However, of late, its use to stabilize the resin-dentin bond is being researched upon so that it can be used in day to day clinical practice. CHX has the potential to bind to both organic and inorganic components of the dentin when it is applied after acid etching on the prepared tooth surface. Thereafter it is not washed off and the dentin bonding agent is applied.
The substantivity of CHX to hydroxyapatite of dentin is believed to be by the formation of a phosphate salt [9]. However, studies in the field of periodontology have also revealed that salivary glycoproteins have an additional role to play in retaining CHX [10]. The mode of interaction of CHX to organic component of dentin is now revealed to be via binding to Type I collagen. The results of our study are in consent with this theory which has revealed the highest substantivity of CHX in dentin discs which were partially demineralized. This implies that CHX got bound to both the organic and inorganic components of dentin in the specimens of this group and hence the better results. This binding of CHX to dentin matrix component is made to advantage in adhesive dentistry as CHX thus bound inhibits the collagen bound proteases and thus exhibits its antiproteolytic action, which in turn enhances the life span of adhesive bonded restorations.
It has already been analysed that the binding of CHX to partially demineralised dentin is better as compared to the totally demineralised one and so the same protocol was applied to this lab test as well [11].
It seems worthwhile to analyse that at which concentration, CHX can best be made use of for enhancing the longevity of bonded restorations
0.2% of CHX was chosen for the study for the fact that at this concentration, it exhibits definite anti MMP-2, -8 and -9 activity [12] and at the same time forms a relatively stable monolayer of retained CHX on the dentin in such a way that an additional increase in the percentage of CHX if used, would provide just an over saturation and not provide any further clinical advantage. However some studies have also mentioned the use of 2% CHX for the similar purpose[7]. Both the concentrations were used in this study in a similar way and the results when interpreted fell in favour of 0.2% CHX.
Therefore, as far as the clinical application is concerned, it is recommended to apply 0.2 %CHX to acid etched dentin, followed by application of dentin bonding agent. Thus the CHX gets sandwiched between the dentin and the adhesive. This phenomenon is responsible for the longevity of the bond of CHX treated hybrid layers [13, 14, 15, 16].
Conclusion
Within limits it can be concluded from our study that 0.2% CHX has a better tendency to bind to partially demineralized dentin and thus can be used clinically to enhance the stability of dentin-adhesive interface.
References
1. Siqueira Jr JF. Paiva SS Rocas IN. Reduction in cultiviable bacterial population in infected root canals by a chlorhexidine based antimicrobial protocol. J Endodont 2007;33:541-7
2. Cosyn J, Wyn I, De Rouck T, Sabzevar MM. Long term clinical effects of a chlorhexidine varnish implemented treatment strategy for chronic periodontitis. J Periodontol 2006;77:406-15
3. de Amorim RG, Leal SC, Bezerra AC, de Amorim FP, de Toledo OA. Association of chlorhexidine and fluoride for plaque control and white spot lesion remineralization in primary dentition. In J Paediatr Dent 2008
4. Carrilho MR, Carvalho RM, de Goes MF, di Hipolito V, Geraldei S, Tay FR et al. Chlorhexidine partially preserves long term dentin bond strength in vitro. J Dent Res 2007;86:90-4
5. Stanislawczuk R, Amaral RC, Zander-Grande C, Gagler D, Reis A, Loguercio AD. Chlorhexidine containing acid conditioner preserves the longevity of resin-dentin bonds. Oper Dent 2009;34:481-90
6. Basrani B, Santos JM, Tjaderhane L, Grad H, Gorduysus O et al. Substantive antimicrobial activity in Chlorhexidine treated human dentin. Oral surg oral med oral pathol oral radiol endod 2002; 94: 240-5.
7. Breschi L, Cammeli F, Visintini E et al. Influenece of Chlorhexidine concentration on the durability of etch-and-rinse dentin bonds: a 12 month in vitro study. J Adhes Dent 2009; 11:191-8
8. Carrilho MR, Carvalho RM, Sousa EN, Nicolau Jose et al. Substantivity of chlorhexidine to human dentin. Dent mat 2010;26:779-85
9. Misra DN. Interaction of chlorhexidine Digluconate with and adsorption of chlorhexidine on hydroxyapatite. J Biomed Mater Res 1994;28:1375-81.
10. Rollla G, Loe H, Schott GR. The affinity of Chlorhexidine for hydroxyapatite and salivary mucins.J Periodontal Res 1970;5:90-5
11. Evaluation of substantivity of Chlorhexidine to human dentin and its application in adhesive dentistry: an in vitro analysis. Indian J Dent 2011;2(2): 8-10
12. Gendron R, Grenier D, Sorsa T, Mayrand D. Inhibtion of the activities of matrix metalloproteinases 2,8 and 9 by chlorhexidine. Clin Diagn Lab Immumol 1999;6:437-9
13. Carrilho MR, Geraldeli S, Tay F, de Goes MF, Carvalho RM et al. In vivo preservation of the hybrid layer of chlorhexidine.J Dent Res 2007;86:529-33.
14. Brackett MG, Tay FR, Brackett WW, Dib A, Dipp FA, Mai S et al. In vivo chlorhexidine stabilization of hybrid layers of an acetone based dentin adhesive. Oper Dent 2009;34:379-83.
15. Hebling J, Pashley DH, Tjaderhane L, Tay FR. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. J Dent Res 2005; 84:741-6.
16. Brackett WW, Tay FR, Brackett MG, Dib A, Sword RJ, Pashley DH. The effect of chlorhexidine on dentin hybrid layers in vivo. Oper Dent 2007; 32:107-11. |