Introduction
The desire to have white teeth and thus a more pleasant smile has become an important esthetic need of the patient1.Tooth bleaching has been reaching great popularity as a result of the esthetic demands imposed by society; it has been indicated for teeth discoloured by aging, trauma, endodontic treatment, ingestion of colored foods and beverage, tobacco, and naturally discoloured teeth2.The successful outcome of any of the applied modalities mainly depends on the etiology, diagnosis, and proper selection of bleaching materials and the correct clinical technique3.
The technique of bleaching or whitening teeth was first described in 1877.4 Hydrogen peroxide was thought to be used for the first time in 1884 and has since been established as the most effective bleaching agent because of its unique ability to penetrate the tooth structure5. Later it was McInnes who reported a technique where hydrogen peroxide, hydrochloric acid and ethyl ether were used. This technique has been found to be successful for bleaching the teeth of patients with endemic fluorosis.6
The efficacy of bleaching agents is validated by many in vitro and in vivo studies.7,8 nevertheless, these bleaching agents should be used only after carefully evaluating the adverseeffects on dental tissues1. In fact, the effect of bleachingprocedures on enamel is still controversial and needs tobe elucidated9.Increased frequency of acid exposure tends to alter the total demineralization/ remineralisation amounts resulting in greater amounts of mineral loss.
Although a reduction in enamel microhardness has been reported,10,13 it must be assumed that this alteration reflects not only the bleaching procedure but also the pH of the formulation used7. In fact, some studies have found no significant differences in enamel microhardness after bleaching with hydrogen peroxide in high concentrations.14,15
Recently casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) derived from cow's milk has been reported to reduce the demineralization process of the tooth structure and enhance the remineralisation process16.
Among laboratory studies, surface microhardness measurement is a simple method to determine the mechanical properties of enamel and dentin surface and it is not only related to the loss or gain of the mineral content of the dental structure, but also to the composition of the applied product and their pH values and the presence of other components in commercial bleaching agents1.
Based on these observations the present study was undertaken to investigate the influence of McInnes bleaching agent on enamel microhardness and subsequent remineralisation by CPP-ACP on bleached enamel.
Materials And Methods:
A total of 20 freshly extracted maxillary anterior teeth were selected. The teeth were then cut sagittaly using diamond disc and the buccal surface were impregnated in the cold cure acrylic resin (DPI) facing upwards (Fig 1). Using the plastic moulds the resin were made in to pellets. These specimens were then kept in artificial saliva to prevent dehydration. The samples were then rinsed in water and dabbed dry with absorbent paper and then subjected to baseline microhardness test using Vickers Micro Hardness Tester (AKASHI-MVK-H2).
 | Fig 1: Showing sectioned specimen with buccal surface impregnated in the cold cure acrylic resin facing upwards.
 |
The test specimens were placed on the stage of tester and stabilized (Fig 2). Then area to indent was selected by focusing with 10x objective lens.After this the load of 100 g was applied on the surface of specimen for 30 seconds, never close to any edge of the specimen. The indentation formed was viewed and measured with 10x objective lens. The average microhardness of the specimen was determined from three indentations to avoid any discrepancy, since the enamel surface has a curvature. The procedure was repeated for all the twenty specimens.
 | Fig 2: Showing the test specimen was placed on the stage of Vickers Micro Hardness Tester (AKASHI-MVK-H2).
 |
Bleaching process
After recording the baseline hardness values McInnes bleaching was prepared using mixture of 1 ml of 36% hydrochloric acid (S D Fine Chem. Limited, Mumbai), 1ml of 30% hydrogen peroxide (S D Fine Chem. Limited, Mumbai) and 0.2 ml of diethyl ether (S D Fine Chem. Limited, Mumbai) which is mixed in the ratio of 5:5:1. The mixture was prepared freshly in a dappen dish before each application.
1) First cycle of bleaching: The bleaching agent thus prepared was applied to the enamel surface using a cotton applicator for five minutes (Fig 3). It was then washed under deionized water, damped dry with absorbent paper and then subjected for microhardness. After this, the samples were stored in artificial saliva for 24 hours to prevent dehydration.
2) Second cycle of bleaching: Again after 24 hours, the second application of bleaching agent was carried out as described earlier and the microhardness values were recorded.
 | Fig 3: Showing the application of McInnes Bleaching agent.
 |
Remineralisation process
1) First cycle of Remineralisation: GC Tooth Mousse (Recaldent) was applied with cotton applicator tips on the post bleached samples, every day for seven days with minimum application time of three minutes (Fig 4). The samples were then washed under deionized water, stored in artificial saliva for next seven days and then subjected for microhardness test as described earlier.
2) Second cycle of remineralisation: Following this, GC Tooth Mousse was applied for seven more days and at the end of fourteen days the samples were again subjected for microhardness testing using the same procedure as described earlier.
 | Fig 4: Showing the application of Remineralising gel (GC Tooth Mousse)
 |
Results:
The data obtained from the following test were subjected for statistical analysis. A "P" value of 0.05 or less was considered for statistical significance. The changes in microhardness at different times of assessment were analysed using the "ONE WAY ANOVA".
In this study these comparisons were done between the groups:
1) In the first comparison baseline was compared with bleaching and remineralisation cycles (Table 1).
2) The next comparison was done between the 1st cycle and 2nd cycle of bleaching (Table 2).
3) The third comparison was done between the 2nd cycle of bleaching and cycles of remineralisation (Table 3).
4) The next comparison was done between the 1st and 2nd cycle of remineralisation (Table 4).
The recorded values were then subjected to statistical analysis.
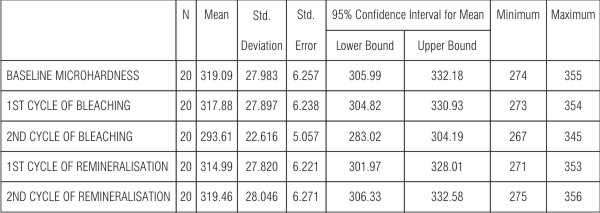 | Table 1 show that baseline microhardness values when compared with first cycle of bleaching decreased by a small margin of 0.4% which was not significant (P=0.034) and a decrease of 4% which was highly significant (P<0.001) when compared with second cycle
 |
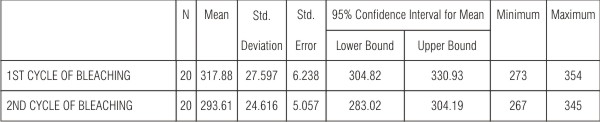 | Table 2 shows the comparison in the microhardness values between the first and second cycle of bleaching which showed a 3% decrease and was significant (P<0.05).
 |
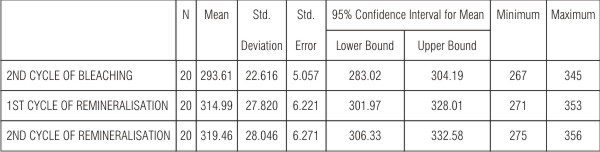 | Table 3 shows 2.8% & 3.5 % increase in Vicker's hardness number and was significant (P<0.01) when the 2nd cycle of bleaching was compared with 1st and 2nd cycle of remineralisation respectively
 |
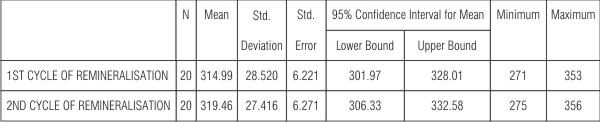 | Table 4 showed an increase in the VHN by 1% and was significant (P<0.05) when comparison was done between first and second cycle of remineralisation
 |
Discussion:
The lightening of the colour of a tooth through the application of a chemical agent to oxidize the organic pigmentation in the tooth is referred to as 'Bleaching'. Bleaching or tooth whitening dates back to the 18th century. In early nineteenth century, hydrogen peroxide alone and in combination with other materials, was used as bleaching agent17.
It was in the year 1966, that McInnes reported a technique that combined hydrochloric acid and hydrogen peroxide to remove fluorosis stains. He used a solution of five parts of 30% hydrogen peroxide, five parts 36% hydrochloric acid and one part diethyl ether and applied the solution with a cotton wrapped toothpick to the areas of the teeth affected by the stain. After 10 to 15 minutes the teeth were washed with water and neutralized with a sodium bicarbonate paste.
It has been reported18 that McInnes bleaching technique were specifically recommended for the treatment of teeth exhibiting endemic dental fluorosis because of its superficial nature, easy manipulation and its quality of being less expensive when compared to other commercially available agents like carbamide peroxide19.In the present study McInnes bleaching agent was selected as it was commonly used in clinical set up for treating dental fluorosis.
The effects of bleaching on enamel microhardness are probably related to their pH, as well as alteration of the organic matrix of enamel under the chemical action of hydrogen peroxide. The strong oxidising effect of hydrogen peroxide on the organic matrix of teeth plays a predominant role in the alterations observed after bleaching10,12. Hydrogen peroxide as diffuses through the enamel and dentin releases free radicals, reactive oxygen molecules and hydrogen peroxide ions that converts long chained dark colored chromophores in to light colored chromophores. This effect can probably be increased by low pH of the bleaching agent, causing subsequent alterations in the mineral composition, decreasing enamel and dentin microhardness20.
All the samples used in the study showed no change in the microhardness immediately after first cycle of bleaching. Around 0.4% reduction in the microhardness was seen which was not significant.
When the bleaching cycle was repeated after 24 hours (the second cycle of bleaching) there was significant reduction in the VHN, which shows 4% decrease in the microhardness, which was significant.
Artificial saliva was used for storing the specimens in between the bleaching cycles, because it is believed that artificial saliva contributed to a slight increase in the microhardness, after demineralisation21.
The amount of demineralisation of enamel by bleaching was assessed by microhardness tester. Microhardness test was selected mainly because it was economical compared to other tests and was easily available22.
Microhardness measurement of tooth material can be done in three different ways like; Knoop's hardness number(KHN), Vicker's hardness number (VHN), andBrinnel's hardness number(BHN).In the present study Vicker hardness number was chosen over Knoop's hardness number because a square shape of indent obtained in VHN was easy and accurate to measure. Even the minute changes in the square shape indent after the test can be easily detected, whereas the Knoop hardness test gave a rhomboid shape indentation with opposing surfaces parallel to each other and detecting the error was difficult.
The average hardness valuefor enamel is in the range from 270 to 350 KHN range or from 250 to 360 VHN range. In this study the microhardness values were in the range from 266.7 to 360 VHN which was within standard range.
One of the factors that affect the hardness measurement was the specimen's preparation, because any tilt or not flat surface would yield a too large an indentation and thus a smaller Vickers's hardness measurement. Hence three indentations were made to avoid any operational bias, then average of these indentations were taken for statistical analysis.
Various techniques have been followed to neutralize the effect of bleaching; the use of baking soda, prophylactic paste containingfluoride, APF gel and use of copious amount of water23. In the present study GC Tooth Mousse has been used, which is commercially available Casein Phosphopeptide (CPP), stabilized amorphous calcium phosphate (ACP) product24.
GC Tooth Mousse was developed by Prof Reynolds at the University of Melbourne in 199825.CPP stabilizes ACP and forms Nano complexes with ACP at the tooth surface thereby providing a reservoir of calcium and phosphate ions which favours mineralization.CPP also buffers the pH of plaque, depresses demineralisation and enhances remineralisation which also results in the anticariogenic property of CPP-ACP26.
The values obtained after the application of remineralizing solution (second cycle of remineralisation) showed an increase in VHN by 1%.
Although most of the remineralizing solutions were supersaturated with respect to amorphous and crystalline calcium phosphate phases, the solutions which were stabilised by the CPP-ACP, such as in GC Tooth Mousse, the spontaneous precipitation of calcium phosphate did not occur and thus the longer the duration of solution in contact with the teeth, the better was the remineralisation27.
According to manufacturer's instructions for the maximum benefit the solutions of GC Tooth Mousse should be left on the tooth surface as long as possible. Hence in the present study the excess GC Tooth Mousse was wiped off after three minutes leaving the residues on the samples and then stored in artificial saliva.
Conclusions:
From the above study it can be concluded that
McInnes bleaching agent does decreases the microhardness of enamel by causing enamel demineralisation.
GC Tooth Mousse used in the study causes an increase in the microhardness of bleached enamel by maintaining high gradient of calcium and phosphate ions at the enamel subsurface.
Refrences:
1. Ameri H, Ghavamnasiri M, Akram A: Effect of different bleaching time intervals on fracture toughness of enamel. J Cons Dent 2011;14(1):73-75.
2. Camargo SEA, Valera MC, Camargo CHR, Mancini MNG, Manezes MM: Penetration of 38% Hydrogen Peroxide into the PulpChamber in Bovine and Human Teeth Submitted to Office Bleach Technique. J Endod2007;33(9):1074-1077.
3. Watts A, Addy M: Tooth discoloration and staining: A review of the literature. Br Dent J.2001;190(6):309-316.
4. Suleiman M, Addy M, Macdonald E, Rees JS: The bleaching depth of a 35% hydrogen peroxide based in- office product: A study in vitro. J Dent 2005;33(1):33-40.
5. Goldstein R, Garber D: Complete Dental Bleaching. Chicago,Quintessence, 1991; pp. 2, 12, 75, 94.McEnoy SA.
6. Chemical agents' for removing intrinsic stains from vital teeth, I: Technique development. Quintessence Int. 1989;20:323-8.
7. Sulieman M, Addy M, Macdonald E & Rees JS (2004) A safety study in vitro for the effects of an in-office bleaching system on the integrity of enamel and dentine Journal of Dentistry 32(7) 581-590.
8. Papathanasiou A, Kastali S, Perry RD &Kugel G (2002) Clinical evaluation of a 35% hydrogen peroxide in-office whitening system Compendium of Continuing Education in Dentistry 23(4) 335-344.
9. de Oliveira R, PaesLeme AF &Giannini M (2005) Effect of carbamide peroxide bleaching gel containing calcium or fluoride on human enamel surface microhardness Brazilian Dental Journal 16(2) 103-106.
10. Seghi RR &Denry I (1992) Effects of external bleaching on indentation and abrasion characteristics of human enamel invitro Journal of Dental Research 71(6) 1340-1344.
11. Attin T, Kocabiyik M, Buchalla W, Hannig C & Becker K (2003) Susceptibility of enamel surfaces to demineralizationnafter application of fluoridated carbamide peroxide gels.Caries Research 37(2) 93-99.
12. Pinto CF, Oliveira R, Cavalli V &Giannini M (2004) Peroxide bleaching agent effects on enamel surface microhardness, roughness and morphology.Brazilian Oral Research 18(4) 306-311.
13. Lewinstein I, Fuhrer N, Churaru N &Cardash H (2004) Effect of different peroxide bleaching regimens and subsequent fluoridation on the hardness of human enamel and dentin.Journal of Prosthetic Dentistry 92(4) 337-342.
14. Park HJ, Kwon TY, Nam SH, Kim HJ, Kim KH & Kim YJ (2004) Changes in bovine enamel after treatment with a 30% hydrogen peroxide bleaching agent.Dental Materials Journal 23(4) 517-521.
15. Sulieman M, Addy M, MacDonald E & Rees JS (2004) Theeffect of hydrogen peroxide concentration on the outcome of tooth whitening: An in vitro study.Journal of Dentistry 32(4) 295-299.
16. Panich M, Poolthong S: The effect of casein phosphopeptide-amorphous calcium phosphate and a Cola soft drink on In vitro Enamel Hardness. J Am Dent Assoc 2009;140:455-460.
17. VimalSikri: Tooth discoloration and bleaching. Textbook of operative dentistry 2ndedi.
18. McEnoy SA. Chemical agents' for removing intrinsic stains from vital teeth, I: Technique development. Quintessence Int. 1989;20:323-8.
19. Chen JH, Xu JW, Shing CX. Decomposition rate of Hydrogen Peroxide bleaching agent under various chemical and physical condition. J Prosthet Dent. 1993;69:46-8.
20. Borges AB, Yui KCK, Avila TC'D, Takahashi CL, Torres CRG, Borges ALS: Influence of remineralizing gels on bleached enamel microhardness in different time intervals. Oper. Dent 2010;35(2):180-186.
21. Chow LC, Takagi S, Carey CM, Sieck BA: Remineralisation effects of a two- solution fluoride mouthrinse: An in situ study.J Dent Res 2000; 79(4):991-995.
22. White DJ, Faller RV, Bowman WD. Demineralization and remineralization evaluation techniques--added considerations. J Dent Res. 1992;71:929-33.
23. Lopes GC, Bonissoni L, Baratieri LN, Vieira LC, Monteiro S Jr: Effect of bleaching agents on the hardness and morphology of enamel. J EsthetRestor Dent 2002;14(1):24-30.
24. Araujo EM, Baratieri LN, Vieira LC, Ritter AV: In situ effect of 10% carbamide peroxide on microhardness of human enamel:function of time. J EsthetRestor Dent 2003;15(3):166-173.
25. Shen P, Cai F, Nowicki A, Vincent J, Reynolds EC: Remineralisation of enamel subsurface lesions by sugar-free chewing gum containing casein phosphopeptide-amorphous calcium phosphate. J Dent Res 2001;80(12):2066-2070.
26. Reynolds EC, Cain CJ, Webber FL, Black CL, Riley PE, Johnson IH, Perich JW: Anticariogenicity of calcium phosphate complexes of tryptic casein phosphopeptide in the rat. J Dent Res 1995;74(6):1272-1279.
27. Spalding M, Taveira LA, de Assis GF: Scanning electron microscopy study of dental enamel surface exposed to 35% hydrogen peroxide: Alone, with saliva, and with 10%carbamide peroxide. J EsthetRestor Dent 2003;15(3):154-164. |