Introduction
A dental implant is subjected to a hostile oral environment that undergoes rapid changes in terms of amount & frequency of occlusal load, temperature, pH value and oral hygiene. Peri-implant tissue breakdown can occur from occlusal overload, microbial invasion and biomechanical action. The oral environment may further contribute to the pathological changes in the tissues adjoining a dental implant.
Normal Peri-Implant Mucosa
The mucosal tissues around intraosseous implants consist of a firmly attached band consisting of a dense collagenous lamina propria covered by stratified squamous keratinizing epithelium. The implant-epithelium junction corresponds to the junctional epithelium around natural teeth, whereby epithelial cells attach to the titanium implant by means of hemidesmosomes and a basal lamina. A well keratinized oral epithelium also covers the outer surface of the peri-implant mucosa. In the marginal area it connects with the barrier epithelium facing the abutment part of the implant. The barrier epithelium has a thickness of only a few cell layers and terminates about 2 mm apical of the soft tissue margin. The collagen fibres originates from the periosteum of the bone crest and extend towards the margin of the soft tissue in directions parallel to the surface of the abutment. Collagen fibres are nonattached and run parallel to the implant surface, in the absence of cementum. The absence of periodontal ligaments marks a significant difference between peri-implant tissues and the periodontal tissues.
Peri-Implant Pathology
Pathologic changes in the tissues coming in contact of a dental implant fall under the definition of peri-implant pathology.
Peri-implant mucositis refers to reversible inflammatory reactions in the mucosa adjacent to an implant. A sustained exposure to plaque over a period of 3 months leads to the tissue breakdown that fails to repair. A comparatively small number of fibroblasts present in this particular lesion may not be able to produce enough collagen and matrix during the reparative phase. This reduced build-up results in an additional propagation and spread of the inflammatory cell infiltration in the peri-implant mucosa.
Peri-implantitis is defined as an inflammatory process that adversely affects the tissues around an osseointegrated implant, leading to loss of supporting bone and impaired function. Berglundh et al (2003) found that mucosa contained large lesions with numerous plasma cells, lymphocytes and macrophages. It was further demonstrated that the inflammatory cell-infiltrate consistently extended to an area apical to the pocket epithelium and that the apical part of the soft lesion frequently reached the bone tissue.
Classification Of Peri-Implantitis
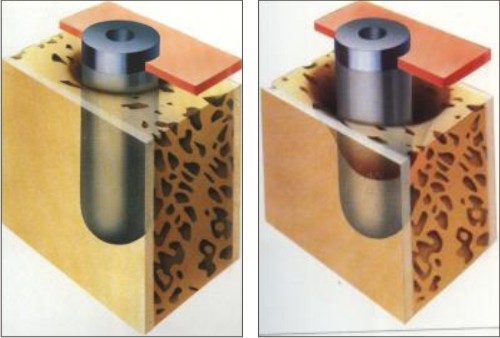
Class 1: Slight horizontal bone loss with minimal peri-implant defects (Fig.1)
Class 2: Moderate horizontal bone loss with isolated vertical defects (Fig.2)
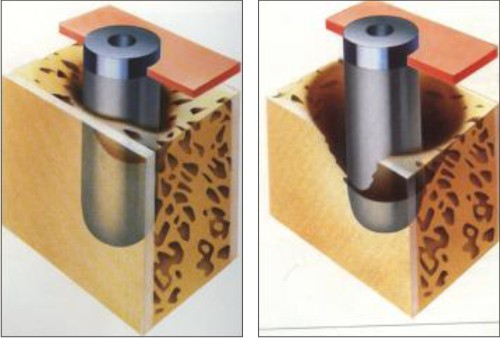
Class 3: Moderate to advanced horizontal bone loss with broad, circular bony defects(Fig.3)
Class 4: Advanced horizontal bone loss with broad, circumferential vertical defects, as well as loss of the oral and/or vestibular bony wall (Fig.4)
 |
 |
 |
 |
Pathogenesis Of Peri-Implantitis
Bacterial flora is associated with periodontitis in natural teeth and peri-implantitis in implant supported prosthesis. The pathogens associated with periodontal disease are a gram-negative, black-pigmented anaerobic flora. Implants with pathological lesions have been clinically characterized by increased mobility, peri-implant radiolucency and probing depths greater than 6mm. Becker et al have demonstrated that the bacteria found in the implant crevice in the successful implant case are basically the same flora as found in the natural tooth crevice/sulcus in a state of health.
Rosenberg et al demonstrated that, in failing implants with a primarily infectious etiology, 42% of the sub gingival flora consists of Peptostreptococcus spp., Fusobacterium spp., and enteric gram-negative rods. Failing implants with a trauma as etiology have a microflora more consistent with gingival health and composed primarily of streptococci.
Etiologic Factors
There are basically two primary etiologic factors that are considered as causative factors in peri-implant bone loss.
1. Biomechanical Overload
Bone loss at the coronal aspect of implants can result from biomechanical overloading and the resultant micro fractures at the coronal aspect of the implant-bone interface. The loss of osseo-integration in this region results in apical down growth of epithelium and connective tissue. The speed and degree of loss of implant-bone contact depends upon the frequency and magnitude of the occlusal loading as well as superimposed bacterial invasion.
The role of over loading is likely to increase in the following four clinical situations:
a. Placement of implant in poor quality bone
b. Orientation and/or number of implants unfavourable for transmission of occlusal load
c. Lack of precision in the fit of prosthetic super-structure and the implant
d. Heavy occlusal load associated with para-functional habits
2. Bacterial Infections
Occlusal loading alone cannot lead to progressive bone resorption. The presence of marginal infection, as in the case of natural teeth is an important etiologic factor. Other etiologic factors such as traumatic surgical techniques, smoking, inadequate amount of host bone resulting in an exposed implant surface at the time of placement and a compromised host response serve as co-factors in the development of peri-implant disease. When plaque accumulates on the implant surface, the sub-epithelial connective tissue becomes infiltrated by large number of inflammatory cells and the epithelium appears ulcerated and loosely adherent. With the apical migration of plaque formation, the clinical and radiographic signs of tissue destruction are seen around both implants and teeth. However the size of the soft tissue inflammatory lesion and the bone loss is larger around Implants. Subsequently, the implant lesions extend into the supra-crestal connective tissue and invade the bone marrow. In addition, different implant surface characteristics influence the amount of peri-implant tissue breakdown and inflammation; specially, HA-coated implants seem to have increased bone loss when compared with titanium implants.
In addition to bacterial infection and excessive biomechanical loading, shape and topography of the implant and the quality of Peri-implant soft tissue attachment play a significant role as etiologic and modifying co-factors in the onset and advancement of the disease.
Management
The long-term objective of the treatment of peri-implant breakdown is to arrest the progress of disease and to achieve an easily maintainable site for the patient. Peri-implant inflammation can be successfully treated by plaque control and effective oral hygiene. Bacterial plaque obtained from implant surfaces is very similar to that removed from natural teeth under both healthy and diseased conditions. Identification of the etiological factors is important for a successful treatment of peri-implantitis. Peri-implant bony defects around functioning implants can be treated through non-surgical or surgical approach.
Biomechanical Overload
In cases of biomechanical forces being the predominant etiologic factors for peri-implant bone loss, treatment is undertaken into two phases.
In the first phase, a critical analysis of the fit of the prothesis, the number & position of the implants and an evaluation of occlusal load should be undertaken. A more favourable change in the number & position of implants, design of the prosthesis and selective occlusal balancing can arrest the progression of peri-implant tissue breakdown.
In order to eliminate deep peri-implant soft tissue pockets or to regenerate bone around the implant, surgical intervention can be employed in a second phase of treatment.
Bacterial Infection
Peri-implant disease caused by bacterial infection should also be treated in phases. The first phase targets to control the acute bacterial infection and to reduce the inflammation present in the tissues. The second phase will involve the surgical procedure. The non-surgical treatment includes mechanical debridement, localized and/ or systemic antimicrobial therapy and improved oral hygiene until a healthy peri-implant site is established.
The Technique
The implant surface gets contaminated with soft tissue cells, bacteria, and bacterial by-products in cases of peri-imolantitis. Bacterial adherence is enhanced by the micro-irregularities of implant surfaces, and as long as the contamination is present, wound healing is compromised. Therefore in order to promote regeneration of new bone and re-osseointegration, the defect must be debrided and implant surface should be made free of any contamination.
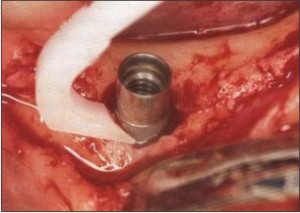
The non surgical treatment of peri-implant bacterial infection involves the local removal of plaque deposits with plastic instruments (Fig.5), polishing of all accessible surfaces with pumice, subgingival irrigation of all periimplant pockets with 0.12% chlorhexidine, systemic antimicrobial therapy for 10 consecutive days and improved patient compliance with oral hygiene until a healthy peri-implant site is established.
 |
 |
The peri-implant pocket epithelium and any granulation tissues are removed using conventional curettes. Care must be taken to avoid damaging or contaminating implant surface. Subsequently plastic curettes are used to remove plaque and calculus as thoroughly as possible from the surface of the implant.
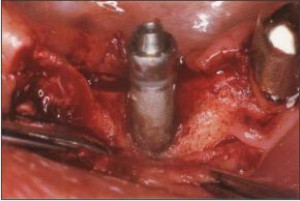
Prophy-Jet Device (30-60 seconds application) is used to clean the implant surface. The Prophy-Jet Device using sodium hydrocarbonate with sterile water are indicated (Bass et al. 1992). The high pressure air powder abrasive used with the equipment removes microbial deposits completely from titanium implant surfaces (Fig.6). In vitro morphologic and statistical
 |
 |
comparisons of gingival fibroblast interactions with titanium surfaces treated with air-powder abrasives showed these surfaces have no adverse effect on cell adhesion.
Consideration should be given to the potential for air-emphysema when using high-pressure air spray instrumentation in the surgical site. Therefore, the spray should never be directed parallel to the implant surface into the surface, but rather at an angle of at least 450 (Brown et al 1992).
The final Step in cleaning the surface of the implant consists of detoxification using citric acid (pH 1-3) for 30 – 60 seconds on a soaked gauze strip around the implant surface. It has been shown that the use of citric acid provides the greatest potential to remove bacteria and endo toxins from the implant surface, in comparison with other chemical agents. (Zablowsky et al. 1992). The entire area should be irrigated again using sterile saline solution, before closing the flap.
Surgical Techniques For Treatment Of Peri-Implantitis
The surgical techniques presently advocated to control peri-implant lesions are modified from techniques used to treat bone defects around teeth. Radiographic evaluation of the site is done to finalize the surgical treatment plan prior to beginning of the procedure. Identification of the defects is done using an explorer under local anesthesia. The whole procedure forms the basis to determine whether the implant will be removed, or a resective type of surgery will undertaken or a regenerative procedure will be adopted.
As in the treatment of certain types of periodontitis, systemic antibiotics have been advocated as a supportive regimen during the treatment phase of peri-implant disease. This may be especially important due to the close proximity of the inflammatory lesions to the implant and the bone marrow. The antibiotics of choice that are used frequently without sensitivity test are doxycycline and metronidazole.
Peri –Implant Resective Therapy
The resective therapy is used to reduce pockets, correct negative osseous architecture & rough implant surfaces and increase the area of keratinized gingiva if needed.
Apically positioned flap techniques and osseous resective therapy are used to correct horizontal bone loss and moderate vertical bone defects and reduce overall pocket depth. Full-thickness or split-thickness flap management is used to access the surgical area (Fig.7 & Fig.8). De-granulation of th e osseous defect is performed after raising the flap. Care should be taken to avoid contact between the implant and metal instruments. Implant surface preparation is performed by applying the air spray of the air-powder abrasive for a maximum of 60 seconds on the implant surface followed by copious irrigation with saline solution. Next, citric acid is applied for 30 seconds followed again by copious irrigation with saline solution.
 |
 |
Implantoplasty
Many times the effort to level the bone and apically position the soft tissues during surgical treatment for peri-implantitis leads to exposure of the rough surface of the implant. Such rough surface tends to accumulate plaque and should therefore be smoothed and polished. Diamond stones with adequate cooling can be used to grind away plasma-spray coatings or threads on the implant surface, with final polishing accomplished using rubber disks (Jovanovic 1990). This type of “implantoplasty” remains the single effective method for reducing plaque accumulation. It also makes plaque control considerably easier for the patient (Lazada et. Al 1990). If this type of implant surface treatment is necessary, it should be performed immediately after flap reflection and before any contouring of the bone. Metal particles always result from this procedure and must be removed by copious irrigation.
Peri-Implant Regenerative Therapy:
The regenerative therapy is also used to reduce pockets but with the ultimate goal of regeneration of lost bone tissue. An increasing number of reports have shown successful treatment of peri-implant bone defects around functioning dental implants. To accomplish regeneration of lost bone tissue and re-osseointegration, guided bone regeneration (GBR) and bone graft techniques have been suggested. In several experimental and clinical studies, the GBR principle using a non-resorbable expanded poly-tetra-fluoro-ethylene membrane has been used for healing of bone defects seen at the time of implant placement and also around failing implants.
Isolation of the affected area from the oral environment enhances regeneration of bone. It is therefore recommended to remove the implant prosthesis 4 to 8 weeks prior to the regenerative surgical procedure to allow optimal compliance with oral hygiene procedures and the soft tissue to collapse and heal over the implant site with a newly attached cover screw in place. Thus at the time of regenerative surgery, a more intact soft tissue flap can be helpful to seal off the peri-implant tissues during the healing period.
A crestal incision is used for the flap design. The surgical therapy includes implant surface preparation by air-powder abrasion for 30-60 seconds and treatment of the site with cotton pledges soaked in citric acid solution (pH 1.0) for 2-5 minutes. Consecutively, an elaborate irrigation of the surgical area is performed with water. Citric acid has been shown to remove endotoxins and bacteria in vitro. A membrane is then trimmed to extend 2-3 mm beyond the margins of the bone defect with a 3mm hole punched in the rigid centre of the membrane that allows attachment to the fixtures. A suture is passed through the portion of membrane and is tied to bend the membrane creating a space beneath. The flap is positioned coronally and sutured.
In cases of larger defects a larger graft material (de-mineralized freeze-dried bone and HA) should be placed to support the membrane. The surgical phase is then sutured closely to the implant neck. The surgical phase is supported by the systemic administration of 250 mg tetracycline hydrochloride every 6 hours for one week. The membrane is removed after 4-5 weeks and the patient is placed on a strict maintenance program. The flap is then positioned back coronally to protect the growing tissue and sutured. The sutures are removed after a week. Care must be taken not to disturb the newly formed osteoid tissue while removing the membrane and during repositioning and suturing of the flap.
Conclusion
The type of osseous defect should be identified before deciding on the treatment modality. Elimination of etiological factors plays a significant role in the success of treatment. A supportive antibiotic therapy enhances the recovery and prognosis. A good oral hygiene plays a crucial role in the over all treatment of peri-implantitis. Re-osseointegration can be defined as the growth of new bone in direct contact to the previously contaminated implant surface without an intervening band of organized connective tissue.
References
1. Glickman Irvin : Clinical periodontology 3rd ed.
2. Lindhe Jan : Clinical periodontology and implant diseases 3rd Ed. 1997.
3. Schlunger Saul et al : Periodontal diseases 2nd 1990
4. Abrahamsson I et al : Peri-implant tissues at submerged and non-submerged titanium implants J. clin periodontal 1999, 26:600-607
5. Haas Robert et al : The relationship of smoking on peri- implant tissue – A retrospective study JPD 1996, 76, 6: 592-6.
6. James Robert A : Periodontal considerations in implant dentistry JPD Aug 1973, vol 30, no. 2, 202-209.
7. Jovanovic Sacha A: The management of peri-implant breakdown around functioning osseointegrated dental implants J. periodontology 1993; 64: 1176-1183.
8. Truhlar Richard – Peri-implantitis cause and treatment. Journal of OMFS clinics of North America May 1998, Vol 10, No.2, 299- 306.
9. Weber Hans Peter and Cochran David K: The soft tissue response to osseointegrated dental implants JPD 1998, 79, 79- 89. |