INTRODUCTION
The combined effects of intrinsic and extrinsic colour determine the appearance of teeth. Injury, antibiotic use, fluoride ingestion and aging can all cause intrinsic discolouration .[1,2] Extrinsic discolouration is produced by staining on the tooth surface and salivary pellicle that forms on the enamel. These stains can be removed by mechanical action and controlled to some degree with toothpaste abrasives. [3,4]
Ingestion of high concentration of fluoride during tooth development causes fluorosis, in which affected teeth are discoloured ranging from opacity to dark brown colour with either smooth surface or pitting. Esthetics in such cases is one of the primary reasons for seeking dental treatment. Out of the various treatment modalities available, tooth bleaching is an effective treatment for such cases. In-office tooth bleaching carried out with either superoxol or 35% carbamide peroxide offers the advantage of rapid colour resolution within limited period as compared to at-home bleaching.
In the present study, two cases having brownish discolouration in the maxillary anterior teeth due to fluorosis were taken up for bleaching. The first patient was treated with superoxol (30% hydrogen peroxide). The second patient was treated with 35% carbamide peroxide (Opalescence Quick).
MATERIALS AND METHOD
In this study two commercially available bleaching agents Opalescence Quick (Ultradent Product Inc, USA) and Superoxol (SD Fine Chemicals, India) were used.
In both the patients, maxillary anterior teeth, comprising of central incisors, lateral incisors and canines were bleached. Oral prophylaxis was first carried out in both the patients.
In the first patient, a 22 year old female, bleaching was carried out with 35% Carbamide peroxide. Impression of the maxillary arch was made using alginate impression material. Cast was poured with dental stone. Bleaching tray was fabricated on the cast using vaccum forming machine, after placing 0.5 mm thick spacer,0.5 mm away on the labial surfaces of all the six maxillary anterior teeth,0.5mm away from the gingival margin. The tray was trimmed and tried in the patient’s mouth. Before the bleaching treatment, carbamide peroxide gel was activated by passing the syringe, containing the gel in running hot water for 1 minute. The gel was placed in the tray and the tray was seated in the patient’s mouth. Excess, extruded gel, if any, was wiped off with cotton gauze and Vaseline was placed on the adjacent gingival tissue and lips. The tray was kept in mouth for 30 minutes after which it was removed and teeth wiped off for bleaching gel.
In the second patient, a 20 year old female, bleaching was carried out with Superoxol (30% hydrogen peroxide). Six maxillary anterior teeth were isolated with rubber dam. Cotton swabs of the same size as the labial surface of the teeth saturated with superoxol
were placed on the labial surfaces of all the six teeth. Heat was applied to these swabs with a spatula after heating on a spirit lamp, so as to activate the hydrogen peroxide solution. After every 10 minutes, Superoxol was replenished to the cotton swabs and reactivated by heat application with a spatula. In this manner the patient was treated for 30 minutes after which the rubber dam was removed.
Both the patients, were given 3 bleaching treatment sittings, at an interval of 1 week in between each sitting.
For evaluation of the colour changes, photographs under standardized conditions were taken before starting the bleaching treatment and after each sitting. The photographs were taken with a Cosina C1 C s/a SLR 35 mm camera, using 35-70 mm, 1:3.5-4.8 zoom lens, to which close up filter of +2 diopter was fitted. Photographs were taken at a film speed of 100 with shutter speed of 1/125 aperture. Value of F/11, focussing ring set at Macro 1.5X from a distance of 1 ½ feet under the same lighting condition i.e. using the same flashlight of Hanimax X140 grade. Fuji ISO 100 film was used for photographs. All the photographs were taken in the same film. The film was developed and printed at Kodak Colour Laboratory.
Evaluation of the colour change was carried out by a dissociated, impartial person on the basis of the photographs giving a grade of either 0 or 1, where 0 indicated No colour change,while 1 indicated significant colour change. Photograph taken after each bleaching treatment sitting was compared with the previous sitting photograph for colour change and graded.
RESULT
In the first patient, treated with 35% Carbamide Peroxide, the colour change scored after the first sitting was 1, second sitting was 1,and at the end of third sitting 1.
In the second patient, treated with Superoxol, the colour change scored after the first sitting was 0, second sitting was 0 and at the end of third sitting was 0.
The colour lightning obtained with 35% Carbamide Peroxide was greater than Superoxol.
DISCUSSION
Bleaching is a long recognized, effective method of restoring normal tooth colour by removing the stains that cause discolouration. Bleaching occurs by oxidation or reduction of the pigments. Whitman et al(5) found Superoxol to be an effective, In-Office bleaching agent. S Chandra and Chawla(6) in a Clinical study found Superoxol to be an effective bleaching agent for fluorosed teeth. O Wellet et al(7), Haywood et al(8,9) Heyman et al(10) in separate studies have found Carbamide peroxide to be an effective bleaching agent. Therefore, for treatment of these two cases, Superoxol (30% hydrogen peroxide) and 35% Carbamide Peroxide were chosen.
Superoxol is 35% hydrogen peroxide by weight and 100% by volume in distilled water. The hydrogen peroxide molecule breaks up and forms oxygen and peridoxyl free radical that fractures the macro-molecular pigments and decolourizes the stain. The resulting molecular products are removed by diffusion. Arwill et al used Na to demonstrate increased enamel permeability after 30% hydrogen peroxide treatment.(11) Crin demonstrated that the peroxide containing bleaching agents could penetrate into the pulp.(12) A certain degree of penetration into enamel and dentin is needed when intrinsic stains have to be removed.
Opalescence quick (35% Carbamide peroxide) is a clear, heavy viscosity, sticky, 35% Carbamide peroxide gel having a pH of 6. Carbamide peroxide gel contains carbopol and urea along with carbamide peroxide, which dissociates into the constituents when it comes into contact with tissue or saliva.(13) Thus carbopol ensures that carbamide peroxide stays active longer in the gel than in a solution. Arendes et al(14) studied the effects of urea on human enamel using SEM, they found that urea was capable of penetrating into the enamel and affecting not only the surface but the interprimate regions of enamel, thus urea penetration can contribute to the permeability of enamel by peroxide and also to structural changes of enamel. Goldberg et al(15) showed that urea attack was mainly interprismatic. Urea is capable of attacking protein structures (by dissociating H-bonds between Co and NH groups). Thus it denatures protein and causes conformational changes. Structural alterations may occur in enamel proteins such as enamelin and amelogenin, thus urea increases the permeability of enamel to peroxides and free radicals. For the same reasons the bleaching effectiveness of 35% Carbamide peroxide gel was found to be more than Superoxol (30% Hydrogen Peroxide) in this study.
1) FIRST PATIENT—BLEACHED WITH 35% CARBAMIDE PEROXIDE
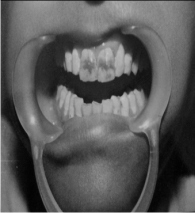 | BEFORE TREATMENT
 |
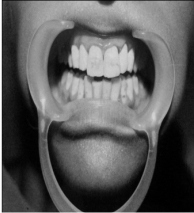 | AFTER TREATMENT
 |
2) SECOND PATIENT—BLEACHED WITH SUPEROXOL (30% HYDROGEN PEROXIDE)
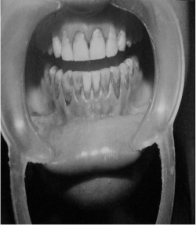 | BEFORE TREATMENT
 |
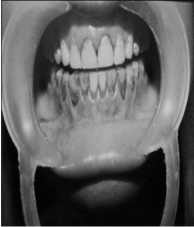 | AFTER TREATMENT
 |
REFERENCES
1. Levy M. Intrinsic tooth discolouration – a review of the literature. Diastema 1976; 4:13-15
2. Watts A, Addy M – Tooth discolouration and staining: a review of the literature. British Dental Journal 2001; 190(6):309-316
3.Addy M, Moran J – Mechanism of stain formation on teeth in particular associated with metal ion and antiseptics. Advances in dental Research 1995; 9:450-456
4. Joiner A, Jones NM, Raven S.J – Investigation of factors influencing stain formation utilizing an in situ model. Advances in Dental Research 1995;9:471-476
5. Whitman FJ, Simon JF – A clinical comparison of two bleaching systems. Journal of the California Dental Association 1995;23(12)59-64
6. Chandra S, Chawla TN – Clinical evaluation of heat method for bleaching of discoloured mottled teeth. JIDA 1974:46(8):313-318
7. Qwellet D, Loe S, Case H, Healy R – Double blind whitening night guard study using 10% carbamide peroxide 1992;4(3):79-83
8. Haywood UB, Leonard RH, Nebox CF, Brusou WD – Effectiveness, side effects and long term status of night guard bleaching 1994;125(9):1219-26
9. Haywood UB, Robinson FG- Vital tooth bleaching with Nightguard vital bleaching current opinion in cosmetic dentistry 1997;4:45-52
10. Heyman HO, Swift EJ Junior, Rayane SC, May KN Jr, Wilder AD Jr, Mann Gb, Peterson CA – Clinical evaluation of two carbamide peroxide tooth whitening agents. Compendium of Continuing Education in Dentistry 1998;19(4):359-362, 364-366,369
11. Arwill T, Myberg N, Soremark R - Penetration of radioactive isotopes through enamel and dentin. Odontologicsk Revy 1969;20:47-54
12 . Crinn GA – Post operative bleaching effect on microleakage. American Journal of Dentistry 1992;5:109
13. Haywood and Heymann HO – Night guard vital bleaching: how safe it is? Quintessence International 1991:23:515-523
14. Aereuds J, JOgenbloed WL et al – Interaction of urea and human enamel. Caries Research 1984;18:17-24
15. Goldberg M, Areuds J, Jogenbloed WL et al – Action of urea solution on human enamel surfaces. Caries Research 1983;17:106-112
|