Introduction
Antimicrobial treatments in Periodontics range from mechanical debridement of tooth surfaces and home plaque removal to local and systemic delivery of chemical antimicrobial agents. Systemic administration has been useful in treating periodontal pockets, but repeated, long-term use of systemic antibiotics is fraught with potential danger including resistant strains and superimposed infections. Inability to achieve and maintain therapeutic concentrations of the drug in the periodontal pocket, risk of adverse drug reactions and dependence of patient compliance are some of the disadvantages reported.1
Local administration, therefore, provides a useful answer to these problems.
Goodson et al in 1979 first proposed the concept of controlled delivery in the treatment of periodontitis. The first delivery devices involved hollow fibers of cellulose acetate filled with tetracycline. They were primarily local delivery devices with minimal control of drug release.2
Pharmacokinetic Parameters In The Periodontal Pocket
Goodson (1989) has pointed out that to be effective in vivo a pharmacological agent must reach its site of action and be maintained there at a sufficient concentration long enough for the intended phar-macological effect to occur. These three criteria (site, concentration and time) are strictly correlated. 3
I. Site Of Action
Gaining access to the anatomical boundaries of the pocket does not necessarily mean gaining access to the target bacteria. Highly complex bacterial biofilm may impair diffusion of and/or inactivate a significant proportion of the applied active agent, and thus protect biofilm bacteria from the action of the antimicrobial.
II. Concentration
To be effective in vivo, an intrinsically efficacious pharmacological agent should reach the site of action at a concentration higher than its minimal efficacious concentration.
However, it is well recognized, that when bacteria are part of highly organized biofilms, significantly higher concentrations are needed.
III. Time
Once a drug reaches the site of action at an effective concentration, it must remain at the site long enough for its pharmacological effect (bacteriostatic or cidal) to occur.
Periodontal Clearance
Periodontal pockets are constantly flushed with flow of inflammatory exudate and the crevicular fluid which markedly increases with gingival inflammation. Goodson (1989) has estimated that the fluid present in a 5mm periodontal pocket is replaced about 40 times an hour. Such high clearance is the result of a low resting volume (0.5µl), and a comparatively high flow rate (20µl). The expected half-time of elimination of an intracrevicularly placed pharmacological agent is about 1 minute which limits the efficacy of locally applied, nonbinding antimicrobial agents in periodontitis treatment. Some medications have this intrinsic ability to bind to the soft and/or hard tissue walls of the pocket (Substantivity).3,4
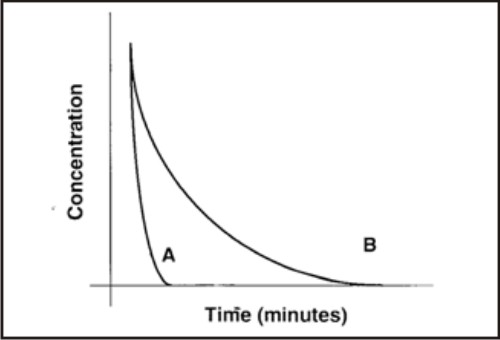 | Clearance of an intracrevicularly placed antimicrobial without substantivity (A) & with substantivity (B).
 |
Indications Of Local Drug Delivery5
There are distinct phases in a periodontal treatment plan where a dental practitioner can use sustained release device
As an adjunct to Scaling and Root planing
Periodontal maintenance therapy: Recurrent periodontitis usually involves only a few teeth. These sites are ideal for the treatment with this device.
Medically compromised patients for whom surgery is not an option or those who refuse surgical treatment.
During periodontal regenerative procedures.
To halt the progression of periodontal disease in patients with moderate periodontitis.
Contraindications
Patients with known hypersensitivity reaction to the antimicrobial used as local drug
Patients susceptible to infective endocarditis who are contraindicated for irrigation devices to avoid the risk of bacteremia
Delivery of antimicrobial using ultrasonic devices is contraindicated in asthmatics, infective conditions such as AIDS, tuberculosis, and those with cardiac pacemakers.
Advantages Of Local Drug Delivery
A local route of drug delivery can attain 100-fold higher concentrations of an antimicrobial agent in subgingival sites compared with a systemic drug regimen thus reduces the total patient dose by over 400 fold. For example, local placement of a tetracycline- releasing ethylene vinyl acetate monolithic fiber can yield tetracycline concentrations in excess of 1300 µg/ml in gingival crevicular fluid over 10 days. (Systemic doses of tetracycline- HCl can provide 4-8 µg/ml in GCF).
Local pocket delivery may employ antimicrobial agents not suitable for systemic administration.
Personally applied antimicrobial regimens offer, for compliant patients, the potential of daily drug placement into periodontal pockets as a part of home self-care procedures.
Professional pocket application of local antimicrobial agents reduces potential problems with the use of systemic antibiotic drug regimens.
It also reduces the risk of developing drug-resistant microbial populations at non oral body sites.5
Disadvantages
-
Difficulty in placing therapeutic concentrations of the antimicrobial agent into deeper parts of periodontal pockets and furcation lesions.
-
Lack of adequate manual dexterity, limited understanding of periodontal anatomy, and poor compliance limits the use of antimicrobial agents by patients as a part of their home self-care procedures
-
Time-consuming and labor-intensive.
-
Nonsustained subgingival drug delivery is limited by only brief exposure of the target microorganisms to the applied antimicrobial agent.
-
Do not markedly affect periodontal pathogens residing within adjacent gingival connective tissues and on extra-pocket oral surfaces (tongue, tonsils and buccal mucosa), which increases the risk of reinfection.
Drugs Used For Local Drug Delivery
A) TETRACYCLINES
Tetracyclines are a group of closely related bacteriostatic antimicrobials. They have been frequently used in treating refractory periodontitis, including localized aggressive periodontitis (LAP).6
Irrigation with Tetracycline
Despite reports of substantivity and anti-microbial efficacy of tetracyclines, mixed results have been reported on the effect of tetracycline irrigation. Subgingival irrigation with 2cc of 5% tetracycline HCI (50 mg/ml) following scaling and root planing and repeated every two weeks for 24 weeks failed to show significant improvement of clinical and microbiologic parameters.
Following a subgingival irrigation for five minutes with a much higher concentration of 50% tetracycline HCI solution retain its activity for 16 days.6
Tetracycline (40%) in petrolatum
40% mixture of tetracycline in petrolatum in the periodontal pocket was used, however no adjunctive clinical benefits to adult and localized aggressive periodontitis patients were seen.
Tetracycline-Containing Fibers
The ACTISITE tetracycline fibres have been approved for the treatment of adult periodontitis both by the United States Food and Drug Administration (FDA) and by the European Union’s regulatory agencies. These are non-resorbable biologically inert, generally considered as safe, plastic copolymer (ethylene and vinyl-acetate) loaded with 25% w/w tetracycline HCI powder packaged as a thread of 0.5 mm in diameter and 23 cm in length. It maintain constant concentrations of active drug in the crevicular fluid in excess of 1000 µg/mL for a period of 10 days. Following application of tetracycline fibres a reproducible depression of the subgingival microbiota has been observed.3
Recently bioresorbable tetracycline fiber has been developed with base of collagen film, which is commercially available as Periodontal plus AB, which offer the advantage of no second appointment for removal as it biodegrades within 7 days.
Tetracycline-Serratiopeptidase-Containing Periodontal Gel Formulation have shown statistically significant results along with scaling and root planing. (Manish Maheshwari 2006)7
Bioerodible Injectable Poly (ortho ester) for Tetracycline Controlled Delivery formulations loaded with tetracycline 10% or 20% showed complete in vitro degradation concomitant with drug release.8
B) Subgingival Doxycycline
The FDA approved 10% doxycycline in a gel system using a syringe (Atridox). ATRIDOX (42.5 mg doxycycline) is a subgingival controlled-release product composed of a 2 syringe mixing system.
Doxycycline levels in GCF peaked to1,500 - 2000 µg/mL in 2 hours following treatment with ATRIDOX. These levels remained above 1000 µg/mL through 18 hours, at which time the levels began to decline gradually. Local levels of doxycycline remained well above the minimum inhibitory concentration for periodontal pathogens (6.0 µg/mL) through Day 7. Approximately 95% of the polymer will bioabsorb or be expelled from the pocket naturally in 28 days. 9
C) Subgingival Minocycline
For minocycline three modes of local application (film, microspheres, and ointment) have been applied clinically.10,11
Film:
Film of ethyl cellulose containing 30% of minocycline cast from ethanol chloroform or chloroform with polyethylene glycol as sustained release device causes complete eradication of pathogenic flora in 14 days.
Microsphere:
The FDA recently approved a new, locally delivered, sustained-release form of minocycline microspheres (ARESTIN) for subgingival placement. The 2% minocycline is encapsulated into bioresorbable microspheres (20-60µm in diameter) in a gel carrier and has resorption time of 21 Days. Gingival crevicular fluid hydrolyses the polymer and release minocycline for 14 days or longer; eventually bioresorb completely.
Ointment:
The Dentomycin (2% gel) minocycline ointment has received regulatory approval for the treatment of periodontitis in the European Union. The same product is available in Japan with the name Periocline. The concentration of minocycline in the periodontal pocket is about 1300µg/ml, 1 hr after single topical application of 0.05 ml ointment (1mg of minocycline) and decreased to 90µg/ml after 7 hr.
D) Subgingival Chlorhexidine
Periocol
Periocol (Eucare Pharmaceuticals, Chennai) is prepared by incorporating 2.5mg chlorhexidine from a 20% chlorhexidine solution in collagen membrane. Size of the chip is 4x5 mm and thickness is 0.25 - 0.32 mm and 10 mg wt.
Collagen is a natural protein, which is chemotactic for fibroblasts, enhances fibroblast attachment via its scaffold like fibrillar structure and stimulates platelet degranulation, thereby accelerating fibers and clot attachment. They resorb after 30 days; however their coronal edge degrades within 10 days.12
Periochip: 2.5 mg Chlorhexidine Gluconate
PerioChip, the controlled subgingival delivery of chlorhexidine gluconate, is a small, orange-brown, tombstone-shaped chip (4.0x 0.5x 0.35mm) in a biodegradable matrix of hydrolyzed gelatin has been approved by FDA. Studies with PerioChip showed reduction in the numbers of the putative periodontopathic organisms Porphyromonas gingivalis, Prevotella intermedia, Bacteroides forsythus, and Campylobacter rectus after placement of the chip. No overgrowth of opportunistic organisms or other adverse changes in the oral microbial ecosystem were noted.
PerioChip releases chlorhexidine in vitro in a biphasic manner, initially releasing approximately 40% of the chlorhexidine within the first 24 hours, and then releasing the remaining chlorhexidine in an almost linear fashion for 7–10 days.
Chlo-Site
Chlo-Site is an agent containing 1.5% chlorhexidine of xanthan type (Ghimas Company, Italy). Xanthan gel is a saccharide polymer, which constitutes of a three-dimensional mesh mechanism, which is biocompatible with chlorhexidine.
The gel gets vanished from the pocket within l0-30 days of injection and effective concentration of chlorhexidine against microorganisms is established for at least 15 days in the region. Both chlorhexidine and gel matrix are muco adhesives so that they stick inside the pockets and are not easily washed out by gingival fluid or saliva. It degrades spontaneuosly at the site of application and is well tolerated and is efficient in treatment of periodontal pockets & peri-implantitis.12
E) Subgingival Metronidazole
A topical medication ELYZOL contains an oil-based metronidazole 25% dental gel (glyceryl mono-oleate and sesame oil). It is applied in viscous consistency to the pocket, where it is liquidized by the body heat and then hardens again, and forming crystals in contact with water.
Studies have shown that metronidazole gel is equivalent to scaling and root planing but have not shown adjunctive benefits with scaling and root planing.96,97 After application of Elyzol 25% dental gel, metronidazole concentrations of above 100 µ/ml were measurable in the periodontal pocket for at least 8 hours and concentrations above 1 µ/ml were found at 36 hours.14
F) Irrigation
In 1966, the first powered irrigation device was introduced in the United States. Since this time, the popularity of oral irrigation subsided; however, recently its potential value in nonsurgical periodontal therapy has been reexamined. The aim of any type of irrigation is to attack the loosely attached plaque both supragingivally and subgingivally. In patients with periodontal diseases, irrigation has been used in two distinct ways. In the therapy phase, irrigation with an antimicrobial may be delivered in the office setting by a dental professional, generally as an adjunct to scaling and root planing. In the maintenance phase daily home irrigation with water or an antimicrobial agent may be added to routine oral hygiene (brushing and flossing).
The efficacy depends upon the penetration, concentration and duration of irrigation. Divergent responses regarding bacterial suppression preclude selection of an ideal duration of therapy or irrigation frequency.15
Adverse Reactions Associated With Local Drug Delivery
An advantage of local delivery is the likelihood of reducing the incidence of adverse effects associated with systemic administration. However, local delivery of antimicrobial agents requires the same caution as the systemic use of drugs.
1. It should not be used in subjects with a known allergy to the active drug.
2. Local drug delivery does not preclude the possible selection of resistant bacterial strains in the pocket or the overgrowth of intrinsically resistant organisms. To date, there is a paucity of data addressing the issue of an increase in bacterial resistance following local delivery of antimicrobials.
3. Possibility of insurgence of oral candidiasis.
4. Local adverse events such as
pain on insertion of the delivery device,
development of abscesses,
tooth sensitivity, patient acceptability, and
interference with taste and other subject related outcomes should be considered.
In general, the use of antibiotics for the treatment of periodontal disease should be highly selective because of potential problems with bacterial resistance. Questions concerning how long the effects of therapy will exist remain to be answered. How these new products will compare to SRP or surgery over a 2- to 3-year maintenance period is yet to be determined. Clearly, further investigations are required. Study lengths and sample sizes must be increased before any concrete conclusions can be drawn regarding the short- or long-term benefit of local drug delivery systems, and provide evidences that these products could be of value in treating periodontal diseases.
Future Perspective
In the future several approaches could be attempted to increase the efficacy of locally delivered drugs against biofilm bacteria, avoiding the adverse effects associated with mechanical instrumentation of the root surface. These approaches include the simultaneous delivery of surface acting agents, enzymes, electric fields, or ultrasounds.
Future therapeutic considerations might include alternating drugs, using different antimicrobials from one treatment time to the next, as more drugs and delivery systems become available. Timed-release, serially-delivered, or combination local delivery drugs, such as metronidazole plus amoxicillin and ketoprofen, may be beneficial for certain refractory subjects. Incorporation of anti-infectives with anti-inflammatory products, as well as growth factors to enhance cell attachment, would, of course, be ideal. Future products should consider incorporation of antimicrobials and other agents into the membranes and guided tissue devices that can enhance regenerative outcomes.
References
1. Offenbacher, S.; Periodontal diseases: pathogenesis. Annals of Periodontology, 1996, 1, 821–878.
2. Kenneth S. Kornman; Controlled-Release Local Delivery Antimicrobials in Periodontics: Prospects for the Future; J Periodontol 1993; 64:782-791.
3. Maurizio S. Tonetti; The topical use of antibiotics in periodontal pockets; Proceedings of the 2nd European workshop on Periodontology.
4. I.G.Needleman; Controlled drug release in Periodontics; A review of new therapies; Br Dent J 1991; 170; 405-408.
5. J Max Goodson; Antimicrobial strategies for treatment of periodontal diseases. Periodontol 2000, 1994; 5; 142-168.
6. Position Paper; The role of controlled Drug Delivery for Periodontitis. J Periodontol 2000; 71; 125- 140.
7. Manish Maheshwari, Gunjan Miglani, Amita Mali et al; Development of Tetracycline-Serratiopeptidase-Containing Periodontal Gel: Formulation and Preliminary Clinical Study; AAPS PharmSciTech 2006; 7 (3) Article 76
8. K. Schwach, P. J. Loup, N. Castioni et al; Bioerodible Injectable Poly(ortho ester) for Tetracycline Controlled Delivery to Periodontal Pockets: Preliminary Trial in Humans; AAPS PharmSci 2002; 4 (4) article 20.
9. ATRIDOX® (doxycycline hyclate) 10% drug description on RxList
10. Shreenivas Akula, Vijay Chava; Minocyclines in periodontal therapy; JISP 2000, vol. 3, no.2, 49-51.
11. Vandekerckhove, Quirynen M, V Steenberghe et al; The use of locally delivered minocycline in the treatment of chronic periodontitis- A review of the literature. J Clin Periodontol 1998;25: 964-968.
12. Nanda Kumar P.K.; Local Drug Delivery-Periocol in Periodontics. Trends Biomater Artif Organs 2006; 19(2);74-80.
13. Kinge B., Attstrom R., Karring T. et al; Three regimen of topical metronidazole compared with subgingival scaling on periodontal pathology in adults. J Clin Periodontol 1992; 19: 710-712.
14. Elyzol 25% dentagel by Product of Colgate-Pamolive Ltd.
15. Kathleen Hodges; Concepts of Non surgical periodontal therapy. 1st edition |