Introduction:
Oral environment is dynamic and the composition of the microbiota may be altered by dietary and nutritional fluctuations as well as changing physical parameters such as eruption and loss of teeth, insertion of dentures, sloughing of epithelial cells, routine dental treatment, antibiotics and radiation therapy (1). Dental plaque is the soft non mineralized bacterial deposit which forms on teeth and dental prosthesis that are not adequately cleaned (2). Plaque formation defined as a complex metabolically interconnected highly organized bacterial ecosystem. It consists of dense masses of microorganisms embedded in an intermicrobial matrix. Dental plaque occurs as a distinct supragingival and subgingival microbial colonization. The next step in plaque development after the formation of pellicle is a selective bonding of certain microorganisms, Streptococcus (S. Sanguis, S. mutans, S. mitior, S. milleri or S salivarius). Actinomyces viscosus is often predominant gram positive filamentous rods. However, A naeslundii, A. israelii1 and Rothia dentocariosa are commonly isolated (3, 4, 5 and 6). Veillonella parvula or genetically similar V. alcalescens are the anaerobic gram negative cocci most commonly encountered in plaque (7). In prosthetic dentistry C. Albicans is commonly accepted as the main causative organism in denture stomatitis and almost invariably found in high numbers in this particular disease (8). The most common oral mucosal disorder in denture wearer is denture stomatitis. The condition is usually associated with the presence of the yeast Candida although several other organisms may be present, after accumulation of Candida cells in the unique microenvironment provided by a denture, a strong immunological reaction occurs and denture stomatitis develops (9, 10, 11, and 12). Streptococcus mutans is the main causative factor on plaque formation which is the main factor in the pathogenesis of denture stomatitis (13 and 14). Denture sore mouth, chronic atrophic candidiasis or more correctly denture stomatitis describes pathogenic changes found in denture bearing tissues (15). The control of denture plaque, bacteria and yeasts by denture hygiene is far better in prevention of denture induced stomatitis than antimycotic drug therapy (16 and 17). Topical antifungal drugs have the drawback of requiring frequent and prolonged administration with relatively large doses and a subsequent risk of systemic adverse experiences (18). Cancer patients are especially prone to oral infection secondary to immunosuppression resulting from the disease itself, chemotherapy and/or radiotherapy and oral dryness. Food sticks more easily to dry mucosa and dry denture surfaces (19). Treatment should include improved denture and oral hygiene which are essential in reducing oral complications of patients undergoing either radiation or chemotherapy (19 and 20). Clinical and laboratory procedures during prosthodontic treatment and denture fabrication should be directed to provide a denture with smooth and homogenous fitting surface. This will facilitate denture cleaning as one of the hygienic measures (21 and 22).
Objective:
The aim of this study is to compare the effect of two different methods of curing acrylic resins, conventional heat curing and microwave heat curing methods on bacterial accumulation on the fitting surface of maxillary obturator.
Material and Methods:
Selection of subjects after the approval of the study from the Ethical Committee. Ten male patients consecutively selected according to the following inclusion and exclusion criteria.
Inclusion criteria:
1- All the subjects are males.
2- Age ranging from 45 to 65 years old.
3- Partially edentulous.
4- Maxillary defect not crossing the midline of the palate Fig. (1).
5- Radiotherapy dose less than 5000 centigray.
Exclusion criteria:
1- Smokers.
2- Subjects with history of wearing obturator.
3- Tumor recurrence.
4- Patients have systemic metabolic diseases.
5- Decreased interarch space.
6- Sever bony undercuts.
7- Sharp or wiry ridges.
 | Fig. 1. Maxillary Defect Not Crossing The Midline Of The Palate
 |
Patients grouping:
Patients were divided into two groups of five patients each:
Group I: receiving acrylic obturator processed by conventional heat curing method.
Group II: receiving acrylic obturator processed by microwave energy.
All patients underwent complete dental and oral examination and intensive dental care.
The same steps for obturator construction were made for both groups except that:
(I).For the first group obturators were cured with conventional heat curing method.
(II).For the second group obturators were made from a mixture of polymer and microliquid monomer* in plastic flasks** made of glass fiber reinforced polyester resin and polycarbon bolts designed especially for microwave use. The investment*** microstone gypsum material was used and the obturators were cured with microwave heat curing method with a built-in turntable. The flasks were placed on their sides on a rotating table while being polymerized in the microwave oven****. The obturators were polymerized initially at 90w for 13 minutes followed by 450w for 2 minutes. Flasks were bench cooled for 21/2 hours to reach room temperature.
All the obturators for the two groups were hollowed, finished and polished Fig. (2) and Fig. (3).
 | Fig. 2. Overview Of The Finished Hollow Obturator
 |
 | Fig.3. hollow obturator inside the patient mouth
 |
All patients of the first and second groups were given a sheet of oral hygiene instructions and were trained on insertion and removal of the obturators and how they keep the obturator and defect area clean.
Bacteriological Procedures:
For the two groups, bacteriologic samples were collected to identify and quantitate microorganisms in the following manner:
1: Before the delivery of the obturator, two swabs were taken from each patient, one from the nasal surface of the surgical cavity and one from the palatal surface. The two samples were considered as a base line (control samples) for each patient of the two groups.
2: After delivery of the obturator, three swabs were taken one from the nasal surface of the surgical cavity, the second from palatal surface of the oral cavity underneath the obturator and the third from the obturator surface facing the surgical cavity. These swabs were taken after one month and after six months from each patient of the two groups for follow up. Each swab was immediately inoculated into a tube containing 1ml. saline. After good shaking, this 1ml. was added to 9ml. saline making a dilution of 1:10. The previous step was repeated to reach a dilution of 1:1000, then 0.1 ml. was transferred from the last dilution (1:1000) and plated onto two nonselective blood agar medium plates (0.1 ml. per plate). One plate was incubated aerobically and the other was incubated anaeropically Fig. (4). The plates which incubated aerobically were incubated for 24 hours in a conventional air incubator. The plates which incubated anaeropically, were incubated for three days at 37 0C using the gas pack anaerobic system Fig. (5). Total viable bacterial count (in 1 ml.) was calculated by the number of colonies X10X103.
 | Fig. 4. One Plate Was Incubated Aerobically And One Anaeropically
 |
 | Fig. 5. The Gas Pack Anaerobic System
 |
3: The colonies on aerobic plates were identified by their morphology, type of haemolysis, microscopic appearance, and biochemical reaction. Different types of colonies on primary isolation anaerobic plates were characterized and identified by their morphology, production of pigment, and haemolysis, as well as microscopic appearance.
4: Colonies which appeared on anaerobic plates only, were confirmed to be pure isolates of obligate anaerobes, to be further identified by a group of biochemical tests which included susceptibility to special potency antibiotic discs, nitrate reduction test as well as growth in 20 % bile.
Data for bacteriological evaluation during different follow up periods were tabulated and statistically analyzed
*H.D. - Justi Co.
**FRP flask (Miracle, H.D. Justi Co., Oxnard, Calif.)
***Whip-Mixcorp, Louis ville, KY)
****Siemens-Electro Gerate-GMB-Made in UK.
Results
Table (1): Shows the numbers of aerobic and anaerobic microorganisms’ isolates and their percentage in relation to total isolates from swab samples of patients with conventional heat cured obturators during the different follow up periods. The total isolates included the sum of aerobic and anaerobic microorganisms’ isolates. Total isolates count was 516. Aerobic isolates count was 364; its percentage of total isolates was 70.54%. Anaerobic isolates count was 152 its percentage of total isolates was 29.46%.The isolated microflora has been shown to be predominantly bacterial in origin. The most predominant aerobic species were staphylococcal species. Its percentage of total isolates was 23.26%, including Staph. epidermidis and Staph. aureus. Other aerobic isolates, streptococcal species included Strepro. Viridans and B. Haemolytic streptocci and its percentage was 17.98%. Also Gram –ve bacilli included E. coli and Klebsiela. The most predominant anaerobic isolates were pigmented bacteroids and spore form forming +ve bacilli. Candida was also isolated.
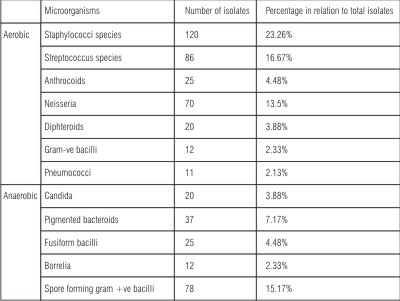 | Table (1): Number of aerobic and anaerobic isolates and its percentage in relation to total isolates from swab samples of patients with heat cured obturators during the different follow up periods:
 |
Table (2): Shows the numbers of aerobic and anaerobic microorganism’s isolates and its percentage in relation to total isolates from swab samples of patients with microwave heat cured obturators during the different follow up periods. The total isolates included the sum of aerobic and anaerobic microorganism’s isolates. Total isolates count was 456. Aerobic isolates count was 330; its percentage of total isolates was 72.37%. Anaerobic isolates count was 126; its percentage of total isolates was 27.63%. The isolated microflora has been shown to be predominantly bacterial in origin. The most predominant aerobic species were staphylococcal were staphylococcal species included Staph. epidermidis and Staph. aureus. Its percentage of total isolates was 25.88%. The percentage of streptococcal species was 17.98%, included Strepto. viridans and B-haemolytic Strepto. Gram -ve bacilli species included E. coli and Klebsiela. The most predominant anaerobic isolates were pigmented bacteroids and spore forming gram +ve bacilli. Candida was also isolated.
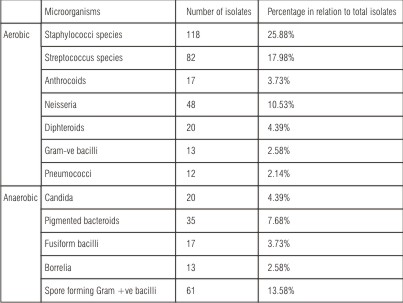 | Table (2): Number of aerobic and anaerobic isolates and its percentage in relation to total isolates from swab samples of patients with microwave heat cured obturators during different follow up periods.
 |
Table (3): Shows the mean of total viable bacterial counts for swab samples of conventional heat cured obturators during different follow up periods. The calculated mean was 31000 before obturator insertion, which showed a gradual increase to be 350000 and 472000, one and six months after obturator insertion.
T-Test procedure used for the comparison of different follow up periods of each group (dependent) and for the comparing between the techniques of the two groups (independent, T-Test). (F.P.S.S. under windows).
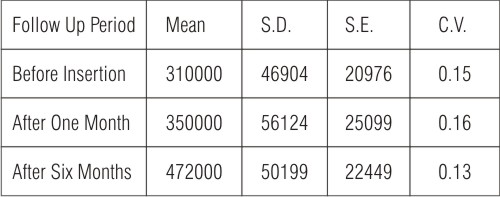 | Table (3): Statistical analysis of the mean of total viable bacterial counts for swab samples of conventional heat cured obturators.
 |
S.D. = Standard deviation.
S.E. = Standard error.
C.V. = Coefficient of variability.
Statistical analysis (Table 4) revealed significant increase between follow up period from before insertion to one month and to six months after insertion and from one month to six months, as P-value is considered significant at P 0.05.
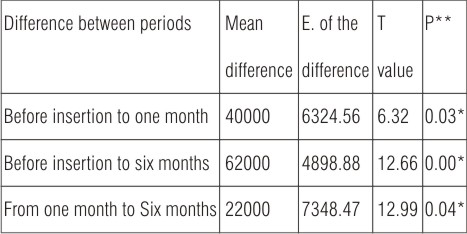 | Table (4): Paired T-Test between different follow up period of conventional heat cured obturators.
 |
*Significant at P<0.05 **P= probability
Table (5): Shows the mean of total viable bacterial counts for swab samples of microwave heat cured obturators during different follow up periods. The calculated mean was 342000 before obturator insertion, which showed a gradual increase to be 376000 and 412000 one and six months after obturator insertion.
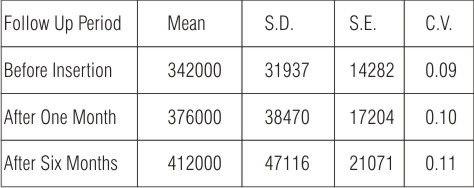 | Table (5): Statistical analysis of the mean of total viable bacterial counts for swab samples of microwave heat cured obturators.
 |
S.D. = Standard deviation
S.E. = Standard error.
C.V. = Coefficient of variability.
Statistical analysis (Table 6) revealed significant increase (P<0.05) between different follow up period before insertion to one month and to six months and form one to six months, as P-value is considered significant at P 0.005.
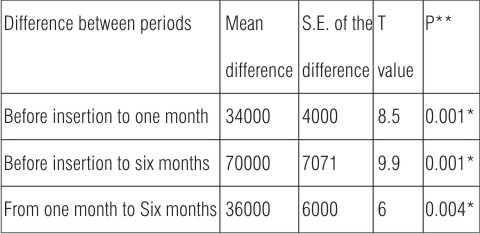 | Table (6): Paired T-test between different follow up period of microwave heat cured obturators.
 |
*Significant at P<0.05 **P= probability
Table (7): Shows a comparison between the mean difference between before insertion and one month after insertion in the conventional heat cured and microwave heat cured obturators techniques.
The calculated mean difference of the conventional heat cured obturators technique was 40000 and the calculated mean difference of the microwave heat cured obturators technique was 34000.
Statistical analysis revealed no significant difference (P>0.05) between before insertion and after one month in the conventional and microwave heat cured techniques, as P-value is considered significant at P 0.05.
 | Table (7): Independent T-test comparing between the mean difference between before insertion and one month after insertion in the two techniques.
 |
*Significant at P<0.05 **P= probability
xx Non significant at P>0.05.
Table (8): Compares between the mean difference between one month and six months after insertion in the conventional heat cured and microwave heat cured obturators techniques.
The calculated mean difference of the conventional heat cured obturators technique was 122000 and the calculated mean difference of the microwave heat cured obturators technique was 36000.
Statistical analysis revealed significant difference at the end of the study between one month and six months after insertion in the two techniques. As the mean difference of the conventional heat cured obturators technique was markedly increased at the end of the study period. Nonparametric Wilcoxon test procedure used for the comparison of two continuous populations when only small independent or dependent samples are available and the population from which they are selected nonnormal (F.P.S.S under windows).
Nonparametric Wilcoxon test procedure used for comparing the difference between before insertion and one month after insertion in the two techniques and the results were:
2- Tailed P= 0.3321xx
Significant at P<0.05
P= probability
xx Nonsignificant at P>0.05
Statistically there was no significant difference between before insertion and after one month in the two techniques.
Nonparametric Wilcoxon test procedure was also used for comparing the difference between one month and six months after insertion in the two techniques and the results were:
2- Tailed P= 0.0084*
*Significant at P<0.05
P= probability
Statistically there was significant difference comparing between one month and six months after insertion in the two techniques.
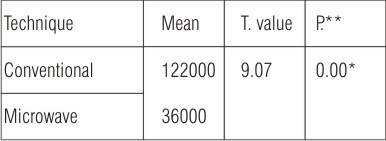 | Table (8): Independent T-test comparing the mean difference between one month and six months after insertion in the two techniques.
 |
*Significant at P<0.05 **P =probability
Discussion Of Results
Samples taken from both groups before insertion of obturators were polymicrobial. The most predominant aerobic microbial isolates were Staph. aureus and Strepto. pyogens and the most anaerobic microbial isolates were black pigmented Bacteroids and spore forming gram +ve Bacilli. Candida was also frequently isolated. Samples were polymicrobial because cancer patients are especially prone to oral infection secondary to immunosuppression resulting from the disease itself Surgery also disturbs normal integrity and function of the mouth anatomically which affects oral flora. In both groups there was statistically significant increase in the total viable bacterial count at the follow up periods after insertion of the maxillary acrylic obturators. This may be attributed to the rough fitting surface of the palatal part of the obturator which favoured plaque formation and accumulation with the alteration of oral environment. The microflora of the obturator plaque was shown to be predominantly bacterial in origin. Staph. aureus was the main dominant kind for aerobic bacteria. This could be attributed to the alterations in saliva and the environment under the obturators. Streptococcal species was detected in a high percentage in both groups because they have the affinity to adhere to oral surfaces. The most dominant anaerobic bacteria in both groups were black pigmented Bacteroids and gram +ve spore forming Bacilli. On comparing between the difference before insertion and after one month in the two techniques there was no statistically significant difference in the total viable bacterial count. On the other hand, six months after insertion of the obturators the difference between the two groups became more apparent and the mean difference of the total viable bacterial count of conventional heat curing group was higher than that of microwave curing ones. The difference was statistically significant. This could be attributed to the extreme decrease of porosity with the use of microliquid monomer which has low vapour pressure even at high temperature with specially formulated with specially formulated crosslinked polymethyl methacrylate for microwave heat curing. While any residual monomer in the conventional heat curing method of acrylic resin obturators causes porosity and dimensional change. Microstone investment was used as its porosity allowed steam to escape and demonstrated fewer crack and holes during microwave heating. Microwave was more efficient in curing non thermally conductive acrylic resin obturators, there was small temperature gradient in the gypsum investment between the edge and the centre during microwave heat curing resulting in superior adaptability. A higher percentage of anaerobic growth distribution was detected in conventional heat curing obturators than in microwave heat cured ones, this can be attributed to the homogeneity and less porosity of the microwave cured obturators than conventional heat cured ones.
Summary And Conclusion
Ten patients with hemimaxillectomy were selected for this study. Patients were divided into two groups (five each). For the first group, patients received heat cured acrylic resin obturators, while patients of the second group received microwave heat cured acrylic resin obturators. Specially formulated cross liked polymethyl methacrylate for microwave heat curing and microliquid monomer was used. The flask for the microwave heat curing was fiber reinforced plastic flask and the investment was microstone gypsum investment. This study was done to evaluate the effect of those two techniques on colonization of bacteria on the fitting surface of the maxillary obturators, palate and the defect. For each patient swab samples were taken for identification of isolates and viable bacterial count before obturator insertion, after one month, and after six months of obturator use. The study revealed that there was an increase in total viable bacterial count after wearing maxillary obturators in both groups between different follow up periods. Comparing between the two groups, there was no significant difference after one month of use of the mean difference of total viable bacterial count statistically. After six months of use, the mean difference of total viable bacterial count in conventional heat cured obturator was greater than that of microwave heat cured obturator and the difference between the two groups was statistically significant.
So it is preferable to process the obturators with microwave heat curing in order to reduce its microbiological effect.
Acknowledgements
I am most thankful to God for all his kindness and grace, for having granted me the patience and perseverance to accomplish the present work. I would like to express my sincere gratitude to Pr.Dr. Alaa, E. Abul Ela for providing me the opportunity to work on this paper under her guidance of which I fell most proud. It was through her kind interest and encouragement that this work was finally achieved. I wish to express my deepest appreciation to Dr. Amal, M. Ibrahim for her valuable advice and suggestions which enabled me to set a plan throughout the course of this work.
References
1- Cole M. and Arnold R. Oral ecology and the normal flora of the mouth. Microbial aspects of oral diseases. N.Y. Pub U.S.A. 1984; pp. 654-59.
2- Loe H., Theilade E. and Jensen S. Experimental gingivitis in man. J. Periodontal 1965; 36: 177-82.
3- Mandel I. Indices for measurement of soft accumulations in clinical studies of oral hygiene and periodontal diseases. 1974; 14: 7-11.
4- Tinanoff N, Gross A and Brady J. Development of plaque on enamel parallel investigations. J. Periodont. Res. 1976; 11: 197-9.
5- Socransky S, Manganiello A, Propas O, Orman V and Vanhoute J. Bacteriological studies of developing supragingival dental plaque. J. Periodont. Res.1977; 12: 90-3.
6- Fine D, Tabak L, Osharin H, Salkind A and Siegel K. Studies on plaque pathogenicity-Plaque collection and limulus lysate, screening of adherent and loosely adherent plaque. J. Periodont. Res. 1978; 13: 17-21.
7- Babb J. Dental Microbiology. N.Y. Int. Pub. U.S.A. pp. 1984; 672-73.
8- Koopmans A, Kalk W and De Graaff J. Efficacy of 2.5% pimafucin suspension in the treatment of denture stomatitis. J. Prosthet. Dent. 1984; 51: 180-2.
9- Walker D, Stafford G, Huggett R and Newcombe R. The ttreatment of denture stomatitis, Evaluation of two agents. Br. Dent. J. 1981; 151: 416-18.
10- Fouche M, Slabbert J and Coogan M. Microorganims isolated from patients with denture stomatitis. J. Dent. Assoc. South Africa. 1986; 41: 313-6.
11- Carter G and Shepherd M. The rational management of oral Candidosis associated with dentures. N.Z. Dent. J. 1986; 82: 81-4.
12- Polsen S, Budtz J, Nielsen L and Kelstrup J. Evaluation of two methods of scoring denture plaque. Acta Odontol. 1988; 41: 288-91.
13- Craige R. Restorative Dental Materials, 13th ED. C.V. Mosoby Co. St. Louis. 1989; 431-56.
14- Nakamoto K and Hamada T. Effects of crude drugs and leberine hydrochloride on the activities of fungi. J. Prosthet. Dent. 1990; 64: 590-2.
15- Jacopino A and Wathen W. Oral candidal infection and stomatitis, A comprehensive review. J.Amer. Dent. Assoc. 1992; 123: 131-35.
16- Theilade E and Theilade J. Formation and ecology of plaque at different locations in the mouth. Scand. J. Dent. Res. 1985; 93: 90-5.
17- Johonson H, Taylor D and Heid W. Clinical evaluation of a nystatin pastille for treatment of denture –related oral candidiasis. J. Prosthet. Dent. 1989; 61: 578-81.
18- Parvinen T, Kokko J and Yli- Urpo A. Micronazole Lacquer compared with jel in treatment of oral cancer. Dental Update. 1994; 231-34.
19- Sweeney M and Bagg J. Oral care for hospice patients with advanced cancer. Dental Update. 1995; 518-22.
20- Carl W. Local radiation and systemic chemotherapy preventing and managing the oral complications. J. Amer. Dent. Assoc. 1993; 124: 119-24.
21- Budtz J. Materials and methods for cleaning dentures. J. Prosthet. Dent. 1979; 42: 619-23.
22- Bergendal T. Treatment of denture stomatitis. A Clinical Microbial and Histological Evaluation. Thesis, Stockholm. 1982. |