INTRODUCTION
Dentists have been perplexed by the problem of tooth discoloration for the last 200 years and have tried numerous chemicals and methods to remove the various types of discoloration The process and outcome of whitening depends upon the cause and type of discoloration. Treatment modalities include micro abrasion, macroabrasion, veneering and placement of porcelain crowns, but they all require cutting of tooth structure. There are increasing numbers of patients who do not want their teeth to be "cut down" for crowns and prefer an alternative, conservative approach of tooth bleaching.
American Dental Association defined bleaching as the treatment, usually involving an oxidative chemical that alters the light absorbing and/or light reflecting nature of the material /structure thereby increasing its value (whiteness)1. The pioneer in the field of bleaching was Macintosh (1799) who invented chloride of lime and named it as "Bleaching Powder". However, from 1860's one of the most effective early techniques for bleaching non- vital teeth was introduced by Truman J.2 using "Labaraque's solution". Since then, several agents were used directly or indirectly to act upon tooth. Considering efficacy, advantages and disadvantages of different materials, most commonly used materials are hydrogen peroxide, carbamide peroxide and sodium perborate
BLEACHING MATERIALS
HYDROGEN PEROXIDE (H2O2) - It is a clear colorless odorless liquid stored in light- proof amber bottles. Various concentrations of this agent are available such as 5.5%, 7.5%, 8%, 10% but 30-35% stabilized aqueous solutions are the most common. Hydrogen peroxide is capable of generating a hydroxyl radical, an oxygen-derived free radical. Hydroxyl radicals are extremely reactive and have been shown to degrade components of connective tissue, particularly collagen and hyaluronic acid. Hydrogen peroxide is caustic and burns tissues on contact, releasing toxic free radicals, perhydroxyl anions, or both. The pH value plays an important role in the rate of reaction in the bleaching process. Ionization of buffered hydrogen peroxide in the pH range of 9.5 to 11.8 produces more perhydroxyl free radicals. The result is a 50% greater bleaching effect in the same time than other pH levels. The average pH value found in various strengths of hydrogen peroxide is approximately 4. This acidity provides hydrogen peroxide a long shelf life. However to achieve efficacy standards, hydrogen peroxide must be buffered to a much higher pH value with a salt of an alkaline base (sodium hydroxide) before being used as a bleaching agent.
Various delivery systems for hydrogen peroxide are:
-
Syringe form
-
Bottle form
-
Strip form- Whitestrips premium- 10% hydrogen peroxide
-
Wrap form- Whitening wrap- 8% hydrogen peroxide
-
Tray -with or without reservoirs.
CARBAMIDE PEROXIDE (CH6 N2 O3)
This agent also known as urea hydrogen peroxide and is available in the concentration range of 3 to 45%. Solutions of 10% carbamide peroxide break down into urea, ammonia, carbon dioxide and approximately 3.5% hydrogen peroxide. Popular commercial preparations contain about 10% carbamide peroxide, with a mean pH of 5 to 6.5. A 35% solution yields 10% hydrogen peroxide. The 15% and a 20% solution of carbamide peroxide are also available for the dentist supervised home bleaching procedure. The 15% carbamide peroxide solution yields 5.4% hydrogen peroxide and the 20% one yields 7% hydrogen peroxide.
A 35% solution of carbamide peroxide is available as to be used by the dentist as in an In Office procedure prior to the patient using the home kit.
It was found that carbamide peroxide has a slower rate of reaction than hydrogen peroxide, especially at room temperature and oral temperatures. Hydrogen peroxide releases oxygen within the first few seconds of contacting surfaces, whereas carbamide peroxide remains active for 40-90 minutes after tissue contact. Oxygen combines with stain molecules in enamel to make stains more soluble and are dissolved into the saliva or an oral rinse.
SODIUM PERBORATE - Na2 [H2 (O2)2 (OH)4 ]
Various types of sodium perborate preparations are available: monohydrate, trihydrate, and tetrahydrate:
-
Sodium perborate monohydrate - active oxygen is 16%
-
Sodium perborate trihydrate - active oxygen is 11.8%
-
Sodium perborate tetrahydrate - active oxygen is 10.4%
This oxidizing agent is available in a powdered form or as various commercial preparationsSodium perborate is stable when dry but, in the presence of acid, warm air or water, decomposes to form sodium metaborate, hydrogen peroxide and nascent oxygen.
MECHANISM OF ACTION OF BLEACHING AGENT:
The whitening mechanism of bleaching is believed to be linked to the degradation of high molecular weight and complex organic molecules that reflect a specific wavelength of light which is responsible for the color of the stain. The resulting degradation products are of lower molecular weight and composed of less complex molecules that reflect less light, resulting in a reduction or elimination of discoloration. Darkly pigmented organic material responsible for enamel discoloration is composed of carbon ring structures with unsaturated double bond.
With further oxidation these products are modified to hydrophillic non-pigmented carbon structures with saturated carbon bonds. Ideally this is the point at which whitening should be terminated. If the degradation process continuous, there is further decomposition of organic matrix, which can lead to complete oxidation with generation of carbon dioxide and water, resulting in a total loss of enamel matrix protein.
BLEACHING TECHNIQUES
These can be classified according to the vitality of the tooth:
1) Non- vital tooth bleaching-
Walking bleach technique
In - office bleaching
Inside/ outside bleaching
2) Vital tooth bleaching-
Dentist- administered bleaching
Dentist- supervised bleaching
Dentist- provided bleaching/ night guard vital bleaching/ matrix bleaching
Over the counter products (OTC)
NON- VITAL TOOTH BLEACHING
Intracoronal bleaching is a conservative alternative to the more invasive esthetic treatment of non- vital discolored teeth. American Assoc. of Endo.1 (1998) suggested that it involves the use of chemical agents placed within the coronal portion of an endodontically treated tooth to remove tooth discoloration. This intracoronal bleaching technique requires the application of at least 2 mm thick layer of protective cement such as zinc polycarboxylate, zinc phosphate, glass ionomer cement, IRM or endodontic obturation.
WALKING BLEACH TECHNIQUE
It is probably the most popular option for bleaching non- vital teeth. Both hydrogen peroxide and sodium perborate have been used for walking bleach technique and various heat sources have been applied to speed up the reaction and improve the bleaching effect. Spasser H.F.3 and Holmstrup G., et al.,4 pioneered the combination of sodium perborate and water or Nutting E.B. and Poe G.S.5 gave the concept of sodium perborate mixed with hydrogen peroxide i.e. Modified walking bleaching technique.
The medicament is placed into the pulp chamber, sealed, left for 3-7 days and is thereafter replaced regularly until acceptable lightening has been achieved. If the tooth has not responded satisfactorily after 2-3 treatments, then Walking bleach technique can be supplemented with an in- office bleaching technique,6 acid etching of dentin internally would open the dentinal tubules to allow better penetration of bleaching agents.7 Various advantages of this technique are that it requires less chair side time, is safer and more comfortable for the patient4.
THERMO/ PHOTO CATALYTIC BLEACHING
A cotton pellet soaked with 30 - 35% hydrogen peroxide solution is placed in the pulp chamber after placing 2mm thick barrier. Heat is applied with a heat or light source with a temperature range of 46-51ºC4.Bleaching should be limited to separate 5- minute's periods rather than a long continuous period8. Light application by specially designed lamps can be used either alone or in combination with heat.
INSIDE/ OUTSIDE BLEACHING TECHNIQUE
Settembrini L., et al.9 gave a modification of walking bleaching technique. This technique lead to reduction in the number of in- office appointments10 In this technique, access to the pulp chamber is gained by the removal of coronal restoration and coronal portion of 2-3 mm of root filling. The remaining root filling is sealed with 2 mm thick glass ionomer cement. The patient places bleaching material, usually 10% carbamide peroxide, intracoronally at regular intervals and covers the lingual aspect of the tooth with a plastic splint (tray). The pulp chamber is left unsealed during the weeks of treatment.The advantage of this method is that it saves chair- side time as bleaching takes place both internally and externally simultaneously11
VITAL TOOTH BLEACHING
DENTIST ADMINISTERED BLEACHING
It requires the use of a high concentration of hydrogen peroxide (30 - 35%) or carbamide peroxide (35-40%). This is often supplemented by a heat or a light source to enhance the action of peroxide12. The technique is accomplished without the anesthesia to determine the patient's pain threshold for the heat level. The main advantages of this technique are that, although it uses caustic chemicals, it is under the complete control of the dentist and the soft tissue is protected from the procedure. The disadvantages of this technique are the cost, the unpredictable nature of the result, chances of pulpal injury and the unknown duration of the treatment.
DENTIST SUPERVISED BLEACHING
It is done by means of a bleaching tray loaded with high concentrations of carbamide peroxide (35-40%) that is placed in the patient's mouth for 30 minutes to 2 hours while the patient is in the dental office.
DENTIST- PROVIDED BLEACHING (Night- guard vital bleaching/Matrix bleaching/Dentist prescribed home - applied bleaching) - This recently introduced bleaching technique has created a resurgence in the field of bleaching primarily because of its relative ease of application, safety of the materials used, the low cost, its general availability to all socio - economic classes of the patients and the high percentage of successful treatment.
It is administered by the patient using 5-22% solution of carbamide peroxide in a custom made tray. Results are generally seen in 2-3 weeks, and the final treatment outcome is complete in 5-6 weeks. However the treatment times may vary extensively and much depends on the amount of time per day the patient chooses or is able to apply the technique. Recently, solutions of hydrogen peroxide that range from 1% to 16% and carbamide peroxide that are either 10% or 15% in concentration have been used.
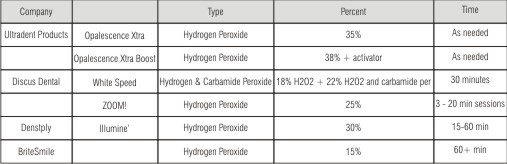 | Brand Professional In-Office Bleaching Products
 |
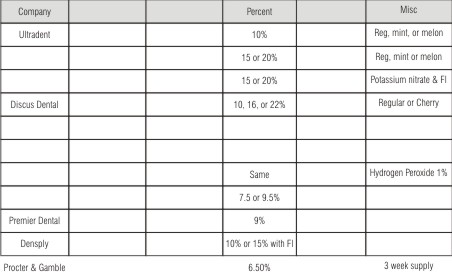 | Few Professional Take Home Bleaching Products
 |
OVER THE COUNTER PRODUCTS- These are often based on carbamide peroxide or hydrogen peroxide of various concentrations and placed in a prefabricated tray.
1. Acid - Rinse: This is usually citric or phosphoric acid which may be harmful to the dentition, as continued rinsing may cause tooth erosion. The potential for misuse may be considerable. The PH of this rinse is between 1 and 2.
2. Bleaching Gel: This gel, applied for two minutes, has an acidic PH of 2-3.
3. Post Bleach 'polishing cream' - This is tooth paste containing titanium di-oxide which may give a temporary painted white appearance.
Whitening strips has been used recently.13
POWER BLEACHING AND IN- OFFICE BLEACHING: Some dentists and patients prefer in-office or power bleaching. A high concentration of hydrogen peroxide (generally 35%) is administered to the teeth with an activating or promoting method (e.g. heat, light, laser) to expedite the whitening effect. The treatment is in complete control of the operator. But it has certain disadvantages. It takes more clinical time and may be more expensive. Longer and more frequent appointments may be needed. Teeth are dehydrated initially which can lead to false evaluation of actual shade change. There are serious safety conditions. The bleach is normally a stronger, caustic concentration and so is more dangerous.
COMPRESSIVE BLEACHING TECHNIQUE
It was suggested that the power bleaching technique could be made more effective by compressing the bleaching material on the tooth. He recommends using 35% hydrogen peroxide in a bleaching tray, seating the tray edges with light cured resin to prevent damages to the soft tissue. The benefit of this method is that it influences the penetration of oxygen ions into the tooth enamel, which improves tooth shade significantly14.
DUAL - ACTIVATED TECHNIQUE- This bleaching system is formulated for both light and chemical activation. Dye indicators have also been incorporated in these bleaching systems.
LASER-ACTIVATED BLEACHING - Reyto R.15 introduced lasers for extracoronal bleaching. The whitening effect with the use of laser is achieved by a chemical oxidation process. Laser bleaching has the advantage that it acts as a jump- start for difficult cases by helping to remove difficult stains such as tetracycline and flourosis.
EFFICACY OF THE BLEACHING TECHNIQUES
INTRINSIC TOOTH BLEACHING
There are various methods and materials available for bleaching non- vital teeth. 90%-95% success with the thermocatalytic bleaching or Walking bleach method has been observed.
Earlier authors reported that the bleaching efficacy of sodium perborate is enhanced by the addition of 30% hydrogen peroxide16,5 but later on it was found that there was no difference in the shade of teeth bleached with sodium perborate in 30% hydrogen peroxide, sodium perborate in 3% hydrogen peroxide or sodium perborate in water.8Some authors suggested that though bleaching efficacy of sodium perborate is enhanced by the addition of 30% hydrogen peroxide, but 30% hydrogen peroxide has been associated with external root resorption when used intracoronally. So. it is recommended that sodium perborate mixed with water rather than 30% hydrogen peroxide or heat should be used in walking bleach method to prevent the occurrence of external root resorption.17
EXTRINSIC TOOTH BLEACHING
Vital tooth bleaching can be performed with a high rate of success as a more conservative measure than restorative treatment, such as porcelain veneers, crowns or composite bonding. It was advocated that most teeth are susceptible to bleaching, provided that the treatment is carried out for a sufficient long time18,19. The first subjective change in tooth color can be observed after 2-4 nights of tooth bleaching using night guard vital bleaching with 10% carbamide peroxide.20 Matis B.A., et al. 21 compared the efficacy and safety of 10% carbamide peroxide to the placebo gel as a home bleaching gel and found that 66% patients had clinically observable color change. Swift E.J. Jr., et al22 in his study examined the use of 10% carbamide peroxide nightly for 2 weeks and found that the teeth were 8 shade units lighter on Vita shade guide. Ritter A.V., et al. 23, from the follow up of 30 patients whose teeth were bleached with 10% carbamide peroxide revealed that 43% patients perceived their tooth color as stable 10 years after bleaching.
Shethri S. Al., et al.24 evaluated two in- office bleaching products (35% and 38% hydrogen peroxide) and found no statistical difference during all active treatment periods and follow - up visits.
Zekonis R., et al.25 compared the efficacy of 10% carbamide peroxide (at- home for 14 days) and 35% hydrogen peroxide (in- office; 60 minutes) and found that 10% carbamide peroxide produced significantly lighter teeth than 35% hydrogen peroxide during all active treatments and follow- up visits. Dietschi D., et al.26 supported this result by his study in which home bleaching using 10%, 15%, 16% and 20% carbamide peroxide was compared to in- office 15% and 30% hydrogen peroxide bleaching and found that home bleaching regime proved more efficient than in- office technique. However the use of higher concentration of carbamide peroxide (15, 16, and 20%) did not prove significantly more effective than 10% carbamide peroxide.
Some studies when compared the bleaching strips (6.5% hydrogen peroxide) and the bleaching tray(10% Carbamide peroxide) and found that bleaching strips were more or equally efficient.27,28,29
ADVERSE EFFECTS
Tooth sensitivity- Tooth sensitivity was found to be the most common side- effect of external tooth bleaching30. It has shown that there is concentration dependent peroxide penetration in the enamel and dentin that entered the pulp chamber31,32 and penetration of the restored tooth was higher than that of intact tooth.33 Also it was found that where heat was used to accelerate the bleaching process and the exposure was 30 minutes thrice, 78% patients suffered sensitivity to cold and intermittent spontaneous pain lasting up to 1 day after treatment34 . Gonzalaz Ochoa J.G. histologically evaluated human pulp after bleaching over night with 10% carbamide peroxide and revealed inflammatory changes in 4 out of 12 teeth after 4 and 14 days' treatment but there was no inflammation in teeth that were bleached with carbamide peroxide for 14 days followed by the recovery phase of 14 days. 35Tooth sensitivity normally persists for up to 4 days after the cessation of bleaching treatment
Cervical root resorption is an inflammatory - mediated external resorption which can be seen after trauma and following intra- coronal bleaching. It was presumed that the irritating chemical diffuses via unprotected dentinal tubules and cementum defects36 Diffusion of hydrogen ions from intra coronal bleaching agents may provide an acidic environment that is optimal for osteoclastic activity and bone resorption. Also the difference in the osteoclastic activity of hydrogen peroxide, carbamide peroxide and sodium perborate is due to the difference in the pH of 35% hydrogen peroxide (3.7), 35% carbamide peroxide (6.5) and sodium perborate (9.9).37
Alterations in the tooth - Several studies on the adverse effects of non- vital bleaching show alterations in the structure and microhardness in the enamel, dentin and cementum. Some studies found that hydrogen peroxide causes denaturation of dentin which may then act as a foreign body and become susceptible to resorption.38 others demonstrated that 35% hydrogen peroxide cause morphological and structural changes in dentin and enamel. These changes are time -dependant and affected directly by pre- treatment with acid etchants.Rotstein I., et al.40 found that 30% hydrogen peroxide reduces the organic component of dentin and cementum, making them more susceptible to degradation while 2% sodium perborate in water did not cause any change in the percentage of inorganic composition of dentin and cementum. They found that dentin and cementum became 10- 12- fold less soluble when sodium perborate mixed with 3% hydrogen peroxide was used instead of 30% hydrogen peroxide.
Alterations in microhardness of enamel and dentin- Despite the widespread use of tooth whitening products, their effects on the physical properties of tooth is still a matter of concern. It was reported that enamel surface developed varying degree of surface porosity and alteration due to bleaching with 10% carbamide peroxide after pretreatment with 37% phosphoric acid41. It was found that the total mineral content of enamel was reduced from 90.39 to 86.01 following bleaching with 10% carbamide peroxide gel. Also the calcium - phosphate ratio decreased from 2.10 to 2.07.42 Also bleaching enamel with 10% and 20% carbamide peroxide could significantly reduce its ultimate tensile strength within 14- day treatment43
To compensate the altered microhardness of enamel, it was assumed that microstructural alterations in bleached enamel may be reversed due to repair mechanism by salivary components. Increase in enamel microhardness could be observed if fluoride, potassium nitrate or sodium citrate is present in the bleaching agent. It was suggested that fluoride treatment following bleaching reduces the demineralization of enamel caused by hydrogen peroxide and carbamide peroxide.44
Effect on restorations- Various studies found that that despite the utilization of modern dentin adhesive systems, non- vital tooth bleaching has shown to adversely affect the sealing ability of resin composites when bonding is performed45,46,47 immediately following bleaching.
A number of studies have examined the reason for reduced bond strength of resin composite to tooth and suggested that the poor bonding surfaces are created by changes in enamel structure resulting from loss of calcium, increased porosity, decrease in microhardness and alterations in the organic substance, manifesting itself with an over- etched appearance and loss of prismatic form.45Another suggested reason could be the residual peroxide from the bleaching agent which interferes with resin attachment and inhibits resin polymerization.48
To compensate the problem of compromised bond strength of resin composites, different treatment options have been investigated. Some authors recommended delays in restorative procedures to avoid clinical problems associated with compromised bond strength following bleaching.49. Others suggested that the removal of superficial enamel after bleaching restores the bond strength of resin composite to the tooth to the normal level. Also use of alcohol- based bonding agents and treatment of access cavities and pulp chambers with 10% sodium ascorbate for 3 hours following bleaching has been recomended50 It was also suggested that 1- week delay in the restorative procedures after bleaching improved the reduced sealing ability of resin composite, but did not reverse it entirely. They found that placement of a calcium hydroxide base and glass ionomer cement over the root canal filling is an important step to seal the root canal from the bleaching agent.
Various studies documented increase in amount of mercury released varied with the type of amalgam and the type of the bleaching agent. It ranged from 4 times to 30 times higher than the saline controls51
It was also found that bleaching may increase the solubility, surface degradation and fluoride release of glass ionomer cement and other cements.52 Also it significantly reduces the microhardness of composites and poly acid modified composites67.
Mucosal irritation- A high concentration of hydrogen peroxide (30-35%) is caustic to the mucous membrane and may cause burns.
Genotoxicity and carcinogenicity of bleaching agents
A dose dependant increased incidence of duodenal hyperplasia where 0.1% and 0.4% hydrogen peroxide was administered to mice via drinking water for 100 days. Benign and malignant lesions in the stomach and duodenum after 90 days exposure were also found.53 However I.A.R.C.54 (the international agency for research on cancer) concluded that there is limited evidence in experimental animals and inadequate in humans for the carcinogenicity of hydrogen peroxide.
Toxicity Of Hydrogen Peroxide And Carbamide Peroxide
The acute effects of hydrogen peroxide ingestion are dependent on the amount ingested and the concentration of the hydrogen peroxide solution. Accidental ingestion of 35% hydrogen peroxide has resulted in several fatal or near-fatal poisonings55. These individuals vomited, were cyanotic, and experienced convulsions and respiratory failure. Young children are at high risk for accidental ingestion. One syringe (3.5 g) of 18% carbamide peroxide yields 210 mg of hydrogen peroxide. Fatal poisoning is therefore not likely even if one ingests one syringe of bleaching agent. Ulceration of the oral mucosa and esophagus, nausea, vomiting, and sore throat have been reported for other hydrogen peroxide-containing preparations. It is therefore important to keep syringes with bleaching agents out of the reach of children, to prevent any possible accident.
REFRENCE
1. American Association of Endodontists.Glossary of Contemporary Terminology for Endodontists 1998; 6th edition: Chicago: 7.
2. Truman J. Bleaching of non-vital discoloured anterior teeth. Dent Times 1864; 1: 69-72.
3. Spasser H.F.A simple bleaching technique using sodium perborate. NYS Dent J 1961; 27 (8): 332-334.
4. Holmstrup G., Palm A.M. and Lambjerg-Hansen H. Bleaching of discoloured root-filled teeth. Endod Dent Traumatol 1988; 4: 197-201.
5. Nutting E.B. and Poe G.S.A new combination for bleaching teeth. J So Calif Dent Assoc 1963; 31 (9): 289-291.
6. Baratieri L.N., Ritter A.V., Monteiro S. and Vieira L.C. Nonvital tooth bleaching: guidelines for the clinician. Quintessence Int 1995; 26:597-608
7. Howell R.A Bleaching discoloured root-filled teeth. Brit Dent J 1980; 148: 159-162.
8. Rotstein I., Chaim M. and Friedman S. Prognosis of intracoronal bleaching with sodium perborate preparations in vitro: 1-year study. J Endod 1993; 19 (1): 10-12.
9. Settembrini L., Gultz J., Kaim J. and Scherer W. A technique for bleaching non vital teeth: inside/outside bleaching. J Am Dent Assoc 1997; 128: 1283-1284.
10. Caughman W.F., Frazier K.B. and Haywood V.B. Carbamide peroxide whitening of nonvital single discolored teeth: case reports. Quintessence Int 1999; 30:155-161
11. Liebenberg W.H. Intracoronal lightening of Discolored pulpless teeth: a modified walking bleach technique. Quintessence Int 1997; 28: 771-777.
12. Tavares M., Stultz J., Newman M., Carpino E. and Goodson. Light augments tooth whitening with peroxide. J Am Dent Assoc 2003; 134 (2): 167-175.
13. Gerlach R.W.Shifting paradigms in whitening: introduction of a novel system for vital tooth bleaching. Comp Cont Edu Dent 2000; 21 (suppl): S4-S9.
14. Miara P. An innovative chairside bleaching protocol for treating stained dentition: Initial Results. Prac Periodont Aesthet Dent 2000; 12 (7): 669-676.
15. Reyto R.Laser tooth Whitening. Dent Clin North Am 1998; 42 (4): 755-762.
16. Ho S. and Goerig A.C. An in vitro comparison of different bleaching agents in the discolored tooth. J Endod 1989; 15: 106-111.
17. Lim M.Y., Lum S., Lee R.S.C. and Lim K.C. An in- vitro comparison of the bleaching efficacy of 35% carbamide peroxide with established intracoronal bleaching agents. Inter Endodont J 2004; 37: 483-488.
18. Goldstein R.E. In-office bleaching: where we came from, where we are today. J Am Dent Assoc 1997; 128 (Suppl): 11S-15S.
19. Dunn J.R. Dentist-prescribed home bleaching: current status. Compend Contin Educ Dent 1998; 19: 760-764.
20. Tam L. The safety of home bleaching techniques. J Can Dent Assoc 1999; 65: 453-455
21. Matis B.A., Cochran M.A., Eckert G. and Carlson T.J. The efficacy and safety of a 10% carbamide peroxide bleaching gel. Quint Int 1998; 29: 555-563.
22. Swift E.J. Jr., May K.N. Jr., Heymann H.O. and Bayne S.C.Two-year clinical evaluation of tooth whitening using an at-home bleaching system. J Esthet Dent 1999; 11: 36-42.
23. Ritter A.V., Leonard R.H., Caplan D.J. and Haywood V.B. Safety and stability of nightguard vital bleaching: 9 to 12 years post-treatment. J Esthet Rest Dent 2002; 14: 275-285.
24. Shethri S. Al., Matis B.A., Zekonis R. and Stropes M. A clinical evaluation of two in- office bleaching products. Oper Dent 2003; 28 (5): 488-495.
25. Zekonis R., Matis B.A., Cochran M.A.and Carlson T.J. Clinical evaluation of in- office and at- home bleaching treatment. Oper Dent 2003; 28 (2): 114-121.
26. Dietschi D., Rossier S. and Krejci I. In vitro colorimetric evaluation of the efficacy of various bleaching methods and products. Quint Int 2006; 37 (7): 515-526.
27. Kugel G., Aboushala A., Zhou X. and Gerlach R.W. Daily use of Whitening strips on tetracycline- stained teeth: comparative results after 2 months. Compendium 2002; 23 (1): 43-47.
28. Gerlach R.W., Gibb R.D. and Sagel P.A. Initial color change and color retention with a hydrogen peroxide bleaching strip. Amer J Dent 2002; 15 (1): 3-7.
29. Sagel P.A., Jeffers M.E., Gibb R.D. and Gerlach R.W. Overview of a professional tooth-whitening system containing 6.5% hydrogen peroxide whitening strips. Compend Contin Educ Dent 2002; 23: 9-15.
30. Jorgensen M.G. and Caroll B.W. Incidence of tooth sensitivity after home whitening treatment. J Am Dent Assoc 2002; 133 (8): 1076-1082.
31. Thitinanthapan W., Satamanont P. and Vongsavan N. In vitro penetration of the pulp chamber by three brands of carbamide peroxide. J Esthet Dent 1999; 11: 259-264.
32. Benneti A.R., Valera M. and Balducci I. In vitro penetration of bleaching agents into the pulp chamber. Inter Endodont J 2004; 37: 120-124.
33. Gokay O, Yilmaz F, Akin S, Tuncbìlek M. and Ertan R. Penetration of the pulp chamber by bleaching agents in teeth restored with various restorative materials. J Endod 2000; 26: 92-94.
34. Nathanson D. and Parra C. Bleaching vital teeth-a review and clinical study. Compend Contin Educ Dent 1987; 8: 490-498
35. Gonzalez-Ochoa J.G.Histological changes to dental pulp after vital bleaching with 10% carbamide peroxide (dissertation). Indianapolis, IN: Indiana University School of Dentistry 2002.
36. Rotstein I., Friedman S., Mor C., Sommer M. and Bab I. Histological characterization of bleaching-induced external root resorption in dogs. J Endod 1991; 17 (7): 436-441.
37. Lee G.P., Lee M.Y., Lum S., Poh R.S.C. and Lim K.C Extraradicular diffusion of hydrogen peroxide and pH changes associated with intracoronal bleaching of discolored teeth. Inter Endo J 2004; 37: 500-506.
38. Lado E.A., Stanley H.R. and Weisman M.I. Cervical resorption in bleached teeth. Oral Surg Oral Med Oral Pathol 1983; 55: 78-80.
39. Titley K., Torneck C.D. and Smith D. The effect of concentrated hydrogen peroxide solutions on the surface morphology of human tooth enamel J Endod 1988; 14 (2): 69-74.
40. Rotstein I., Lehr Z. and Gedalia I. Effect of bleaching agents on inorganic components of human dentin and cementum. J Endod 1992; 18 (6): 290-293.
41. Bitter N.C. A scanning electron microscopy study of the effect of bleaching agents on enamel: A preliminary report. J Prosthet Dent 1992; 67 (6): 852-855.
42. Goo D.H., Kwon T.Y., Kim H.J., Kim K.H. and Kim Y.J. The efficacy of 10% carbamide peroxide gel on dental enamel. Dent Mater J 2004; 23 (4): 522-527.
43. Cavalli V., Carvalho R.M. and Giannini M. Effect of carbamide peroxide bleaching agents on tensile strength of human enamel. Dent Mater 2004; 20 (8): 733-739.
44. Bizhang M., Seemann R., Duve G., Romhild G., Altenburger M.J., Jahn K.R. and Zimmer S. Demineralization effects of two bleaching procedures on enamel surfaces with and without post-treatment fluoride application. Oper Dent 2006; 31 (6): 705-709.
45. Ben-Amar A., Liberman R., Gorfil C. and Bernstein Y. Effect of mouth guard bleaching on enamel surface. Am J Dent 1995; 8: 29-32.
46. Spyrides G.M. and Perdigao J. Effect of whitening agents on dentin bonding. J Esthet Dent 2000; 12: 264-270.
47. Teixiera E.C.N., Turssi C.P., Hara A.K. and Serra M.C. Influence of post- bleaching intervals on dentin bond strength. Braz Oral Res 2004; 18 (1): 132-136.
48. Timpawat S., Nipattamanon C., Kijsamanmith K. and Messer H.H. Effect of bleaching agents on bonding to pulp chamber dentine. Inter Endo J 2005; 38 (4): 211-217.
49. Torneck C.D., Titley K.C. and Smith D.C. Effect of water leaching on the adhesion of composite resin to bleached and unbleached bovine enamel. J Endod 1991; 17: 156-160.
50. Turkun M. and Turkun L.S. Effect of nonvital bleaching with 10% carbamide peroxide on sealing ability of resin composite restorations. Inter Endo J 2004; 37: 52-60.
51. Rotstein I., Mor C. and Arwaz J.R. Changes in surface levels of mercury, silver, tin and copper of dental amalgam treated with Carbamide peroxide and Hydrogen peroxide in- vitro Oral Surg Oral Med Oral Path Oral Radiol Endod 1997; 83: 506-509.
52. Swift E.J. Jr. and Perdigao J. Effects of bleaching on teeth and restorations. Compend Contin Educ Dent 1998; 19: 815-820
53. Ito A., Naito M., Nayto Y. and Watanaee H. Induction of duodenal tumors in mice by oral administration of hydrogen peroxide Gann 1981; 72: 174-175.
54. I.A.R.C. International Agency on Research on Cancer. Monographs on the evaluation of carcinogenic risks to humans. Re-evaluation of hydrogen peroxide. 1999; Vol. 71. Lyon: IARC.
55. Dahl J.E. Tooth bleaching - a critical review of the biological aspects. Crit Rev Oral Biol Med 2003; 14 (4): 292-304.
|