Introduction
In restorative dentistry materials are mainly utilized to replace dental tissue lost through dental caries and tooth preparation procedures. When a significant amount of tooth tissue is lost the dental pulp may be adversely affected. This may necessitate advanced conservative procedures involving extirpation of the dental pulp and obliteration of the space with gutta-percha and root canal sealers. Access for these procedures is through the tooth crown. Should this be unsuccessful, surgical Endodontics is necessary to allow cleaning and sealing of the root end to prevent further infection.1
Most endodontic failures occur as a result of leakage of irritants into the periapical tissues2,3. An ideal orthograde or retrograde filling material should seal the pathways of communication between the root canal system and its surrounding tissues. It should also be nontoxic, noncarcinogenic, nongenotoxic, biocompatible with the host tissues, insoluble in tissue fluids, and dimensionally stable4,5. Furthermore, the presence of moisture should not affect its sealing ability; it should be easy to use and be radiopaque for recognition on radiographs4. Because existing restorative materials used in Endodontics did not possess these ''ideal'' characteristics4, mineral trioxide aggregate (MTA) was developed and recommended initially as a root-end filling material and subsequently has been used for pulp capping, pulpotomy, apexogenesis, apical barrier formation in teeth with open apexes, repair of root perforations, and as a root canal filling material. MTA has been recognized as a bioactive material6 that is hard tissue conductive7, hard tissue , and biocompatible.
An extensive search of the endodontic literature was made to identify publications related to calcium hydroxide-based root canal sealers. The articles were assessed for the outcome of laboratory and clinical studies on their biological properties and physical characteristics. Comparative studies with other sealers were also considered. Several studies were evaluated covering different properties of calcium hydroxide-based sealers. Calcium Hydroxide based root canal sealers have a variety of physical and biological properties.
Brief Introduction Of Materials
MTA:
In the 1990s a new material, mineral trioxide aggregate (MTA) was developed at Loma Linda University as a root- end filling material. The first publication on the use of the material to seal root perforations was published in 1993. It is commercially available as ProRoot MTA (Tulsa Dental Products, Tulsa, OK, USA).The use of MTA as a root-end filling material was identified due to the fact that the material is hydraulic cement i.e. it sets in the presence of water. Mineral trioxide aggregate (MTA) is now used extensively in Endodontics.
Two commercial forms of MTA are available (ProRoot MTA); namely the grey and the white MTA both with similar chemical and physical properties. MTA is essentially Portland cement (used in the building industry as a binder in concrete) with 4:1 proportions of bismuth oxide added for radiopacity. The material was originally reported to be composed of calcium and phosphate and its biocompatibility was attributed to its similarity to dental hard tissues. However Camilleri and co-workers 4 have shown that MTA is composed primarily of tricalcium and dicalcium silicate, the main constituent elements of Portland cement, which on hydration produce a silicate hydrate gel and calcium hydroxide, not calcium phosphate as claimed by Torabinejad.8
PORTLAND CEMENT:
It is a fine powder produced by grinding cement clinker. It is classified as hydraulic cement, which normally is composed of lime, silica, alumina and ferric oxide. Lime is composed of calcium and magnesium oxides. PC is produced by grinding clay and lime bearing materials in the correct proportions and then heating the mixture to 1400o. This process called calcinations produces physical and chemical changes in the raw materials. The resulting clinker is ground to a fine powder and a small amount of gypsum is added to retard the setting process.9
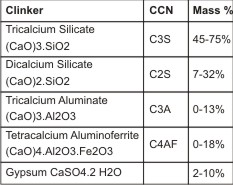 | Typical constituents of Portland clinker plus Gypsum Cement chemists notation under CCN.
 |
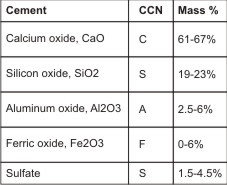 | Typical constituents of Portland cement Cement chemists notation under CCN.
 |
CALCIUM HYDROXIDE:
The first clinical use of calcium hydroxide as a root canal-filling material was probably by Rhoner in 1940.10 It took another 20 years for calcium hydroxide to become popular for apexification, the sealing of perforations, and management of resorption.11 A ''miracle' material'' Biocalex (Laboratoire SPAD, Dijon, France), developed by French researchers, was believed to make radical changes to endodontic instrumentation methods.12 The calcium hydroxide containing a pulp-capping agent, Dycal (Dentsply-Caulk, Milford, DE), also became popular as a sealer among some clinicians in late 1970s.13
The two most important reasons for using calcium hydroxide as a root-filling material are stimulation of the periapical tissues in order to maintain health or promote healing and secondly for its antimicrobial effects. The exact mechanisms are unknown, but the following mechanisms of actions have been proposed:
1. Calcium hydroxide is antibacterial depending on the availability of free hydroxyl ions14,15. It has a very high pH (hydroxyl group) that encourages repair and active calcification. There is an initial degenerative response in the immediate vicinity followed rapidly by a mineralization and ossification response16.
2. The alkaline pH of calcium hydroxide neutralizes lactic acid from osteoclasts and prevents dissolution of mineralized components of teeth. This pH also activates alkaline phosphatase that plays an important role in hard tissue formation17.
3. Calcium hydroxide denatures proteins found in the root canal and makes them less toxic.
4. Calcium hydroxide activates the calcium-dependent adenosine triphosphatase reaction associated with hard tissue formation17, 18.
5. Calcium hydroxide diffuses through dentinal tubules and may communicate with the periodontal ligament space to arrest external root resorption and accelerate healing16, 19.
EVALUATION OF PROPERTIES
1. PARTICLE SIZE:
The physical properties of cement might be influenced by crystal size. Smaller particles increase surface contact with the mixing liquid and lead to greater early strength as well as ease of handling. A recent study reported that some particles of MTA are as small as 1.5 mm, which is smaller than the diameter of some dentinal tubules20. The authors hypothesized that this might play an important role in the sealing ability of MTA after hydration and production of a hydraulic seal. This hypothesis might not be clinically relevant, because the dentinal tubules after root canal instrumentation or root-end cavity preparation are not open unless the operator removes the smear layer by acid-etching these surfaces.
The particle size of MTA is reported in many articles. Lee et al21 reported particle sizes ranging from 1-10 mm for GMTA powder, whereas Camilleri22 reported that the WMTA powder has particles less than 1 to approximately 30 mm before hydration.
The handling characteristic of PC is dependent on its particle size and shape. Many investigations evaluated particle size and shape of MTA20. WMTA has finer particles than 2 types of Pc23.
2. CHEMICAL PROPERTIES:
The MTA patent25 shows that it contains calcium oxide (CaO) and silicon (SiO). Several investigations have reported that the main elemental components of MTA are calcium and silica, as well as bismuth oxide26, 27. MTA is currently marketed in 2 forms: gray (GMTA) and white (WMTA). MTA was introduced in gray, but because of the discoloration potential of GMTA, WMTA was developed24. Investigations showed that lower amounts of iron, aluminium, and magnesium are present in WMTA than in GMTA24.
The primary differences between both types of MTA and PC are a lack of potassium and the presence of bismuth oxide24. An investigation evaluated the dry powder of GMTA and WMTA, as well as ordinary and white Portland cement (PC), finding that all tested materials have similar major constituents: tricalcium silicate, tricalcium aluminate, calcium silicate, and tetracalcium aluminoferrite.
An investigation confirmed the similarity of PC and MTA, except for the presence of potassium and lack of bismuth oxide24. Another study showed lower amounts of aluminium and sulphur in WMTA compared with WPC22.
3. SETTING TIME:
MTA is prepared by mixing its powder with sterile water in a 3:1 powder-to-liquid ratio 39. The mean setting time of MTA is 165_ 5 minutes, which is longer than amalgam, Super EBA, and intermediate restorative material (IRM). GMTA exhibits significantly higher initial and final setting times than WMTA29, 30. The longer setting time of WMTA in comparison with PC is attributed to the lower levels of sulphur and tricalcium aluminate in WMTA23.
In an attempt to use MTA for 1-visit perforation repair, a study placed glass ionomer cement (GIC) over WMTA. The authors reported that placing GIC 45 minutes after WMTA placement did not affect the formation of calcium salts in the interface of the 2 materials. In addition, placement of GIC over WMTA did not affect setting of this material31. A separate study by the same authors confirmed that the GIC setting was not disrupted by the presence of WMTA32.
Dycal has a very short setting time, and in its use as a root canal sealer users recommended first introducing the catalyst paste into the canal with a lentulospiral followed by a gutta-percha cone coated with the base paste13. The setting reactions of calcium hydroxide-containing sealers are complex. Even though the sealer surface becomes hard, the inner mass may remain soft for an extended period. Apexit was reported to have a setting time of less than 2 hours at 100% relative humidity33. CRCS sets within 3 days in both dry and humid environments. Sealapex sets in 2 to 3 weeks in 100% relative humidity and does not set in a dry environment34. Many of its properties support its use alone as a root canal-filling/sealing material, although its placement in canals may be challenging.
4. FLOWABILITY
The setting time of Portland cement and MTA could be reduced by removing the gypsum from the manufacturing process without affecting its other prop-erties. Superplasticizer was used to obtain a homogeneous flowable mix and control setting time ranging from 5 to 12 minutes. Although this material can be applied for dental usage, there are no studies to date concerning the effect of superplasticizer concentrations and liquid-to-powder ratios on properties of the cement.
The mean flows of AWPC plus 1.8% and 2.4% polycarboxylate superplasticizer groups were significantly increased (p < 0.001) at all liquid-to-powder ratios when compared with control groups. Significant differences between AWPC plus 1.2% polycarboxylate superplasticizer group and the control groups were observed only in a 0.33 liquid-to-powder ratio (p < 0.001).
An acceptable flow within the working time is important for any root canal sealer in order to reach and seal the apical foramen and lateral dentinal wall irregularities. Flow depends on particle size, shear rate, temperature, and time from mixing. It can be measured with either a rheometer or from the diameter of the film of sealer between two glass plates under load37. Pitt Ford et al36 reported significant differences in flow between Apexit and Tubliseal EWT, the former being superior in a rheometer test and the latter with the traditional method. In another study, the flow of Apexit was comparable to AH Plus and Tubliseal EWT33.
5. SETTING EXPANSION:
There are conflicting results regarding the setting expansion of various types of MTA29, 30. Two investigations showed that WMTA expands slightly more than GMTA29, 30.
Excessive expansion that might result in a cracked root is an undesirable property when a material is used as a root-end filling substance30. The setting expansion of PC is a matter of controversy in the literature. One investigation reported that both types of WPC and OPC show greater expansion than GMTA and WMTA30. In contrast, another experiment showed that the setting expansion of PC is less than GMTA and more than WMTA38. This might be attributed to the differences of chemical composition among various types of PC.
Biocalex in the presence of moisture in the canal expands by up to 280%. This has the potential to create severe postoperative pain and vertical root fractures. Apexit also has exhibited high water sorption but along with its equally high solubility gives rise to minor overall dimensional change33. CRCS was quite stable with volumetric changes in water for 21 days. Sealapex displayed significant sorption in a 100% humid atmosphere with volumetric expansion.
6. SOLUBILITY:
The degree of solubility of MTA is a matter of debate among investigators8, 30. Most investigations reported low or no solubility for MTA8, 30. However, increased solubility is reported in a long-term study. When comparing the physical properties of WMTA with those of GMTA, the former material demonstrates significantly more solubility30. Varying results are reported when comparing PC with WMTA30. One study reported that both ordinary and white PC (WPC) exhibits significantly less solubility than WMTA30. These findings are in contrast with another study that shows WMTA is less soluble than 2 different types of PC. The differences are attributed to the type of PC used in these investigations.
In addition, the powder-to- water ratio might influence the amount of solubility. In fact, higher water-to-powder ratios increased MTA porosity and solubility42. The authors reported that using more water would increase calcium release from MTA. The addition of bismuth oxide to MTA, which is insoluble in water, is another cause for MTA insolubility. In an experiment on the hydration of MTA, Camilleri22 confirmed the reaction of bismuth oxide with both calcium and silicate contents of MTA.
The solubility in water and 37% phosphoric acid, and compressive strength of four brands of hard-setting calcium hydroxide base materials were studied. Results were highly variable among brands and no correlations appeared to exist between properties studied. One product was significantly different from the others with regard to acid solubility and compressive strength39.
7. COMPRESSIVE STRENGTH:
There are conflicting results regarding the compressive strength of WMTA and GMTA30. One study reported that compressive strength of WMTA at 3 and 28 days after mixing is significantly less than that of GMTA30. In contrast, 2 other investigations comparing the compressive strength of GMTA and WMTA reported more compressive strength for WMTA30. In general, MTA's compressive strength is not significantly affected by condensation pressure42. Another recent experiment revealed that keeping WMTA in dry conditions decreases its compressive strength41. Even the samples kept moist after mixing show variations in compressive strength, de-pending on the amount of time elapsed between mixing and examination. The samples that were kept for 2-7 days in moisture exhibited greater compressive strength than the 4-hour samples. A recent investigation reported significantly lower compressive strength for WMTA when the material was etched by phosphoric acid (37%). The investigators suggested that restoration with acid-etch composite after MTA placement should be postponed for at least 96 hours44.
The compressive strength of some types of OPC and WPC is significantly lower than WMTA and GMTA 28 days after hydration30. Attaining adequate compressive strength is important for some of the clinical applications of MTA such as repairing perforations and pulp capping. These procedures require materials with adequate compressive strength to be stable against occlusal pressure.
The strength of a brittle material depends upon the toughness of the material itself and the degree of perfection with which it can be placed. This study shows that both the compressive and tensile strength of calcium hydroxide lining materials is dependent on the fracture toughness at unstable crack propagation, KIC, and the size, number and distribution of pores (which are the defects that nucleate fracture). Of the products tested here, Dycal possesses the greatest actual strength due to a higher than average KIC value and relatively few isolated small pores40.
8. FLEXURE STRENGTH:
On the basis of limited literature, it appears that placing a moist cotton pellet over MTA for the first 24 hours increases its flexural strength.
9. PUSH-OUT STRENGTH:
The push-out strength of perforation repair materials is an important factor because shortly after perforation repair, tooth function might dislodge the material. MTA has lower push-out strength in comparison with IRM or Super EBA after immersion in walking bleach materials. On the basis of available data, it appears that MTA gains optimal physical properties such as flexural strength, compressive strength, and push-out strength when it receives enough moisture after being placed in an operation site.
10. pH:
The pH value of MTA is 10.2 after mixing. This value rises to 12.5 at 3 hours8. Comparing pH values of GMTA with WMTA, the latter material displays a significantly higher pH value 60 minutes after mixing 29, 30. MTA kept its high pH value throughout the course of a long-term study; the authors attributed the high pH value to the constant release of calcium from MTA and the formation of CH. Comparing pH values at different periods of time, both WMTA and GMTA exhibit significantly higher pH values than 2 types of PC immediately after mixing30. However, 30 minutes after mixing, no statistical difference can be found among the tested materials. At 60 minutes, GMTA has a significantly lower pH value than WMTA and both types of PC30. Available data show that mixing MTA with water results in the formation of CH and a high pH environment.
The pH of calcium hydroxide paste has been shown to be as high as 12.5 when used for intracanal medicament purposes15. In an experimental study, the pH of distilled water in contact with Sealapex reached 11.5 during 30 days, most of which was gained in the first 1 hour, followed by CRCS (10.5), Apexit (10.5), and Sealer 26 (9.5)45. In another experiment, the maximum pH of Sealapex and CRCS in a 1-week study did not exceed 9.1 and 7.8, respectively46. Further research on Sealapex showed a slow and gradual rise in pH (not exceeding 9.57) in bidistilled water in the first hour, after which the sample disintegrated in solution, whereas CRCS did not cross the 7.65 mark47.
11. RADIOPACITY:
The mean radiopacity for MTA has been reported at 7.17 mm of an equivalent thickness of aluminum8. This value is higher than that reported for Super EBA or IRM in a separate investigation. Another study compared the same materials and reported more radiopacity for Super EBA and IRM than MTA. The difference can be due to the use of different methods to evaluate the radiopacity of test materials. Comparing the radiopacity of WMTA with that of GMTA, 2 separate studies reported more radiopacity for WMTA 29, 30. Because a similar amount of bismuth oxide is used to produce radiopacity in both materials, the presence of other substances in WMTA might be the reason for this difference between the two.
The mixtures of Portland cement with bismuth oxide and lead oxide presented the highest radiopacity values and differed significantly from the other materials (p < 0.05), whereas Portland cement/zinc oxide presented the lowest radiopacity values of all mixtures (p < 0.05). Portland cement/bismuth subnitrate and Portland cement/iodoform presented statistically similar radiopacity values (p > 0.05), and both materials were more radiopaque than Portland cement associated with zirconium oxide, bismuth carbonate, barium sulfate, calcium tungstate, and zinc oxide (p < 0.05)48.
According to ISO 6876/2001 standards, the recommended radiopacity of the root canal sealer should be at least that of a 3-mm thick aluminium wedge. Radiographs of samples of Sealapex showed large voids in their structure, and they were less radiopaque than CRCS for 3 weeks, after which the voids disappeared and there was an increase in radiopacity for Sealapex49.
12. POROSITY:
Many studies have evaluated MTA porosity. The amount of porosity in mixed cement is related to the amount of water added to make a cement paste, entrapment of air bubbles during the mixing procedure, or the environmental acidic pH value28.
13. MICROHARDNESS:
The microhardness of MTA can be influenced by several factors such as the pH value of the environment, the thickness of the material, the condensation pressure, the amount of entrapped air in the mixture, humidity, acid etching of the material, and temperature 23, 28, 42, 44. An acidic environment has an adverse effect on the microhardness of both GMTA and WMTA.
The microhardness of 2-mm and 5-mm thicknesses of GMTA and WMTA was investigated when the materials were used as an apical barrier. Regardless of the formulation of MTA or placement technique used, a 5-mm thickness is significantly harder than a 2-mm thickness. An investigation compared the microhardness of WMTA with 2 types of PC. WMTA showed significantly more microhardness than both types of PC49, which can be attributed to the different chemical and physical properties of the tested materials23, 24. A recent study confirmed a trend of less microhardness after using more pressure during MTA condensation42. Two separate investigations reported that EDTA and acid-etch procedure significantly reduce MTA microhardness44.
14. BIOCOMPATIBILITY:
Both MTA and Portland cement are bioactive materials. The biocompatibility of the materials had originally been attributed to the chemical similarity to tooth hard tissues namely calcium phosphate. However this has been shown not to be the case. MTA produces calcium hydroxide as a by product of the hydration reaction. The similarity of action of both MTA and Portland cement to calcium hydroxide had been postulated.
Calcium hydroxide is used extensively in dentistry. When using SEM to study the biocompatibility of dental materials it is imperative to ensure there is no reaction between the material and the reagents used in the experimental procedure. Scanning electron microscopy thus is contraindicated to evaluate cell growth and expression over materials based on Portland cement. Other methods of assessing biocompatibility are thus preferred1.
15. ANTIBACTERIAL PROPERTIES:
In an antimicrobial study on MTA and PC against Staphylococcus aureus, Enterococcus faecalis, Pseudomonas aeruginosa, Bacillus subtilis, Candida albicans, a wild fungus, and a mixture of these bacterial and fungal species, both materials exhibited diffusion in agar without inhibition of microbial growth50. Some investigations showed that GMTA51, 52, 53 and WMTA52 have an antifungal effect. In contrast, others showed that GMTA has limited or no antifungal effect50. One experiment showed that freshly mixed and 24-hour set GMTA have an antifungal effect on C. Albicans51. The antifungal effect of MTA might be due to its high pH or to substances that are released from MTA into the media. In contrast, a study comparing the effect of MTA and PC on C. albicans, S. aureus, and Escherichia coli showed no antimicrobial effect for either of the tested materials54. Another investigation reported antimicrobial activity of GMTA, WPC, and OPC on Micrococcus luteus, S. aureus, E. coli, P. aeruginosa, C. albicans, and Enterococcus faecalis53.
The literature shows that MTA has an antibacterial and antifungal effect. Lowering the powder-to-liquid ratio might adversely affect the antibacterial and antifungal properties of MTA.
The incorporation of antibacterial components in root canal sealers may be an important factor in preventing the regrowth of residual bacteria and controlling bacterial re-entry into the root canal system. The antibacterial effect of calcium hydroxide is based on its ability to release hydroxyl ions and to raise pH.
Conclusion
Both MTA and Portland cement are a bioactive material that influences its surrounding environment. There are many published reports regarding the chemical, physical, and anti-bacterial properties of MTA. This article showed that MTA is composed of calcium, silica, and bismuth. It has a long setting time, high ph, and low compressive strength. It possesses some antibacterial and antifungal properties, depending on its powder-to-liquid ratio. Inspite of its advantages it has a disadvantage of high cost and poor handling.
PC is manufactured widely all around the world, and it is impossible to control the quality, composition, and biocompatibility of these materials. The higher solubility of some types of PC is also a matter of concern. The compressive strength of some types of OPC and WPC is significantly lower than WMTA and GMTA 28 days after hydration. Excessive expansion that might result in a cracked root is an undesirable property when a material is used as a root-end filling material. Despite some similarities between PC and MTA, it is not safe to use PC, which has not been formulated for human use, in place of a bioactive medical material such as MTA.
Calcium hydroxide-based root canal sealers have a variety of physical and biological properties. Comparative studies reveal their mild cytotoxicity, but their antibacterial effects are variable. Further research is required to establish the tissue healing properties of calcium hydroxide in root canal sealers. Calcium hydroxide is used extensively in dentistry. When using SEM to study the biocompatibility of dental materials it is imperative to ensure there is no reaction between the material and the reagents used in the experimental procedure.
Further studies are required to assess properties of these materials for their use as root end filling materials.
References
1. A review of methods used to study biocompatibility of Portland cement derived materials used in dentistry. Malta medical journal 2006: 18: 3: 9-14.
2. Siqueira JF Jr, Roˆ c¸ as IN. Clinical implications and microbiology of bacterial persistence after treatment procedures. J Endod 2008;34:1291-301.
3. Ng YL, Mann V, Rahbaran S, Lewsey J, Gulabivala K. Outcome of primary root canal treatment: systematic review of the literature: part 2-influence of clinical factors. Int Endod J 2008;41:6-31.
4. Torabinejad M, Pitt Ford TR. Root end filling materials: a review. Endod Dent Trau-matol 1996; 12:161-78.
5. Ribeiro DA. Do endodontic compounds induce genetic damage? a comprehensive review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008; 105:251-6.
6. Enkel B, Dupas C, Armengol V, et al. Bioactive materials in endodontics. Expert Rev Med Devices 2008; 5:475-94.
7. Moretton TR, Brown CE Jr, Legan JJ, Kafrawy AH. Tissue reactions after subcutaneous and intraosseous implantation of mineral trioxide aggregate and ethoxybenzoic acid cement. J Biomed Mater Res 2000; 52:528-33.
8. Mahmoud Torabinejad, Physical and Chemical Properties of a New Root-End Filling Material J Endod 1995; 21: 7; 349-353.
9. Understanding mineral trioxide aggregate/Portland-cement: a review of literature and background factors. European Archives of Paediatric Dentistry, June, 2009 by R. Steffen.
10. Leonardo M, Leal J, Filho A. Pulpectomy: immediate root canal filling with calcium hydroxide. Oral Surg Oral Med Oral Pathol 1980;49:441-50.
11. Ingle J, Luebke R, Walton R, et al. Obturation of the radicular space. In: Ingle J, ed. Endodontics. 2nd ed. Philadelphia, PA: Lea & Febiger; 1976:217-77.
12. Hendra L. Biocalex-a new approach to endodontia dependent upon biological principles and chemical action only. J Br Endod Soc 1970; Autumn: 37-41.
13. Goldberg F, Gurfinkel J. Analysis of the use of Dycal with gutta percha points as an endodontic filling technique. Oral Surg Oral Med Oral Pathol 1979;47:78-82.
14. Cvek M. Treatment of non-vital permanent incisors with calcium hydroxide. Odont Revy 1974;25:239.
15. Bystrom A, Claesson R, Sundqvist G. The antimicrobial effect of camphorated para-monochlorophenol, camphorated phenol and calcium hydroxide in treatment of infected root canals. Endod Dent Traumatol 1985;1:170-5.
16. Manhart M. The calcium hydroxide method of endodontic sealing. Oral Surg Oral Med Oral Pathol 1982;54:219-24.
17. Stock C. Calcium hydroxide: root resorption and perio-endo lesions. Br Dent J 1985;158:325-34.
18. Torneck C, Moe H, Howley T. The effect of calcium hydroxide on porcine pulp fibroblasts in vitro. J Endod 1983;9:131-6.
19. Tronstad L, Andreason J, Hasselgren G, et al. pH changes in dental tissues after root canal filling with calcium hydroxide. J Endod 1981;7:17-21.
20. Komabayashi T, Spa° ngberg LS. Comparative analysis of the particle size and shape of commercially available mineral trioxide aggregates and Portland cement: a study with a flow particle image analyzer. J Endod 2008;34:94-8.
21. Lee YL, Lee BS, Lin FH, Yun Lin A, Lan WH, Lin CP. Effects of physiological envi-ronments on the hydration behavior of mineral trioxide aggregate. Biomaterials 2004;25: 787-93.
22. Camilleri J. Hydration mechanisms of mineral trioxide aggregate. Int Endod J 2007;40:462-70.
23. Dammaschke T, Gerth HU, Zu¨ chner H, Scha¨ fer E. Chemical and physical surface and bulk material characterization of white ProRoot MTA and two Portland cements. Dent Mater 2005;21:731-8.
24. Song JS, Mante FK, Romanow WJ, Kim S. Chemical analysis of powder and set forms of Portland cement, gray ProRoot MTA, white ProRoot MTA, and gray MTA-Angelus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;102: 809-15.
25. Torabinejad M, White DJ. Tooth filling material and use. US Patent number 5,769,638; May 1995.
26. Camilleri J, Montesin FE, Brady K, Sweeney R, Curtis RV, Ford TR. The constitution of mineral trioxide aggregate. Dent Mater 2005;21:297-303.
27. Asgary S, Parirokh M, Eghbal MJ, Stowe S, Brink F. A qualitative X-ray analysis of white and grey mineral trioxide aggregate using compositional imaging. J Mater Sci Mater Med 2006;17:187-91.
28. Torabinejad M, Watson TF, Pitt Ford TR. Sealing ability of a mineral trioxide aggregate when used as a root end filling material. J Endod 1993;19:591-5. Dent Res 1983;91:316-9.
29. Chng HK, Islam I, Yap AU, Tong YW, Koh ET. Properties of a new root-end filling material. J Endod 2005;31:665-8.
30. Islam I, Chng HK, Yap AU. Comparison of the physical and mechanical properties of MTA and Portland cement. J Endod 2006;32:193-7.
31. Nandini S, Ballal S, Kandaswamy D. Influence of glass-ionomer cement on the interface and setting reaction of mineral trioxide aggregate when used as a furcal repair material using laser Raman spectroscopic analysis. J Endod 2007;33: 167-72.
32. Ballal S, Venkateshbabu N, Nandini S, Kandaswamy D. An in vitro study to assess the setting and surface crazing of conventional glass ionomer cement when layered over partially set mineral trioxide aggregate. J Endod 2008;34:478-80. after periradicular surgery in cats. J Endod 1998;24:720-5.
33. McMichen F, Pearson G, Rahbaran S, et al. A comparative study of selected physical properties of five root canal sealers. Int Endod J 2003;36:629-35.
34. Ingle J, Newton C, West JG, et al. Obturation of the radicular space. In: Ingle J, Bakland L, eds. Endodontics. 5th ed. Hamilton Ontario, Canada: B C Decker Inc. Ltd.; 2002:571-668.
35. Setting Time and Flowability of Accelerated Portland Cement Mixed with Polycarboxylate Superplasticizer. Norachai Wongkornchaowalit, DDS, and Veera Lertchirakarn, DDS, MDSc, PhD. JOE: 37; 3; March 2011
36. Pitt Ford T, Lacey S, Watson T, et al. A study of the rheological properties of endodontic sealers. Int Endod J 2005;38:499-504.
37. Ørstavik D. Materials used for root canal obturation: technical, biological and clinical testing. Endod Topics 2005;12:25v38.
38. Storm B, Eichmiller FC, Tordik PA, Goodell GG. Setting expansion of gray and white mineral trioxide aggregate and Portland cement. J Endod 2008;34:80-2.
39. Acid and water solubility and strength of calcium hydroxide bases. M Hwas and JL Sandrik. J Am Dent Assoc, Vol 108, No 1, 46-48:1984
40. The strength and fracture toughness of calcium hydroxide preparations. Lloyd CH, Anderson JN. J Oral Rehabil. 1980 Mar;7(2):155-65.
41. Chogle S, Mickel AK, Chan DM, Huffaker K, Jones JJ. Intracanal assessment of mineral trioxide aggregate setting and sealing properties. Gen Dent 2007;55: 306-11.
42. Nekoofar MH, Adusei G, Sheykhrezae MS, Hayes SJ, Bryant ST, Dummer PM. The effect of condensation pressure on selected physical properties of mineral trioxide aggregate. Int Endod J 2007;40:453-61.
43. Holt DM, Watts JD, Beeson TJ, Kirkpatrick TC, Rutledge RE. The anti-microbial effect against enterococcus faecalis and the compressive strength of two types of mineral trioxide aggregate mixed with sterile water or 2% chlorhexidine liquid. J Endod 2007;33:844-7.
44. Kayahan MB, Nekoofar MH, Kazandag M, et al. Effect of acid-etching procedure on selected physical properties of mineral trioxide aggregate. Int Endod J 2009;42:1004-14.
45. Da Silva L, Leonardo M, Silva RD, et al. Calcium hydroxide root canal sealers: evalu-ation of pH, calcium ion concentration and conductivity. Int Endod J 1997;30:205-9.
46. Gordon T, Alexander J. Influence on pH level of two calcium hydroxide root canal sealers in vitro. Oral Surg Oral Med Oral Pathol 1986;61:624-8.
47. Tagger M, Tagger E, Kfir A. Release of calcium and hydroxyl ions from set endodontic sealers containing calcium hydroxide. J Endod 1988;14:588-91.
48. Radiopacity of Portland Cement Associated With Different Radiopacifying Agents. Marco Antonio Hu´ngaro Duarte, J Endod 2009; 35:737-740.
49. Caicedo R, von Fraunhofer J. The properties of endodontic sealer cements. J Endod 1988; 14:527-34.
50. Estrela C, Bammann LL, Estrela CR, Silva RS, Pe´ cora JD. Antimicrobial and chem-ical study of MTA, Portland cement, calcium hydroxide paste, Sealapex and Dycal. Braz Dent J 2000;11:3-9.
51. Al-Nazhan S, Al-Judai A. Evaluation of antifungal activity of mineral trioxide aggre-gate. J Endod 2003;29:826-7.
52. Mohammadi Z, Modaresi J, Yazdizadeh M. Evaluation of the antifungal effects of mineral trioxide aggregate materials. Aust Endod J 2006; 32:120-2.
53. Tanomaru-Filho M, Tanomaru JM, Barros DB, Watanabe E, Ito IY. In vitro antimicrobial activity of endodontic sealers, MTA-based cements and Portland cement. J Oral Sci 2007;49: 41-5.
54. Al-Hezaimi K, Al-Hamdan K, Naghshbandi J, Oglesby S, Simon JH, Rotstein I. Effect of white-colored mineral trioxide aggregate in different concentrations on Candida albicans in vitro. J Endod 2005; 31:684-6. |