|
|
|
| Polymorphous Low Grade Adenocarcinoma: A Case Report |
Meena Kulkarni 1 , Mahesh Gabhane 2 , Aarti Mahajan 3
1 Professor and Head, Department of Oral Pathology and Microbiology, - M.G.V Dental College and Hospital,Nashik, Maharashtra state, India.
2 Post graduate student, Department of Oral Pathology and Microbiology, - M.G.V Dental College and Hospital,Nashik, Maharashtra state, India.
3 Reader, Department of Oral Pathology and Microbiology, - M.G.V Dental College and Hospital,Nashik, Maharashtra state, India.
|
| Address For Correspondence |
Dr. Mahesh Gabhane
A-5, Unity Park Building, Vise Mala, College Road,
Nashik-422005, Maharashtra, India.
Email: dr.mack0385@gmail.com
Ph No: +919890282031 |
| Abstract |
| Polymorphous low grade adenocarcinoma (PLGA) is a malignant epithelial tumor that is essentially limited in occurrence to minor salivary gland sites and is characterized by bland, uniform nuclear features, diverse but characteristic architecture, infiltrative growth and perineural infiltration. It is the second most common salivary gland malignancy, with infrequent recurrence, and not uncommonly showing locoregional metastasis. It is a low grade, indolent neoplasm with good prognosis. This article describes a case of polymorphous low grade adenocarcinoma (PLGA) in the right posterolateral portion of the hard palate, in a 51-year-old woman. |
|
| Keywords |
| Infiltrative growth, Locoregional Metastasis, Malignant Epithelial Tumor, Minor Salivary Gland, Perineural Infiltration. |
|
| Full Text |
INTRODUCTION
Polymorphous low grade adenocarcinoma (PLGA) is a malignant epithelial tumor that is essentially limited in occurrence to minor salivary gland sites and is characterized by bland, uniform nuclear features, diverse but characteristic architecture, infiltrative growth and perineural infiltration. This is a recently recognized type of salivary gland tumor first described in 1983. Evans and Batsakis first used the term polymorphous low grade adenocarcinoma to describe this tumor. Majority of Polymorphous low grade adenocarcinomas were diagnosed as adenocarcinoma not otherwise specified pleomorphic adenomas or adenoid cystic carcinoma. The present case report focuses on the histopathology of Polymorphous low grade adenocarcinoma and its differential diagnosis from pleomorphic adenoma and adenoid cystic carcinoma. (1)
Case Report
A 51 year old female patient visited the department of Oral Pathology and Microbiology, with the chief complaint of swelling on the palate on the right side since the last one year. The history revealed that the swelling had started insidiously and had steadily increased in size since its onset. (Figure 1) The swelling was not associated with aggravating or relieving factors. Medical, surgical, dental, family and personal histories were not noteworthy. There were no abnormalities detected on general physical examination.
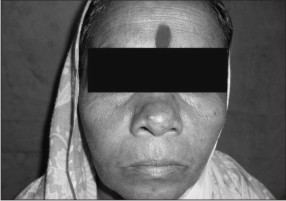 | Fig. 1-: Showing firm and nodular, diffuse swelling over the right Infraorbital region and just besides the lateral wall of nose.
 |
Intraoral examination revealed an ulceroproliferative lesion, involving the right posterolateral region of the hard palate, measuring approximately 5 X 3 cm, extending antero-posteriorly from mesial surface of maxillary first molar to 1 cm posterior to maxillary tuberosity and mesio-laterally from palatal surface of maxillary molars, crossing the midline and extending 0.5 cm to the left side. (Figure 2) On palpation, the swelling was tender
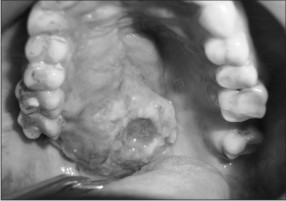 | Fig. 2-: Showing Ulcero-proliferative lesions with irregular border on lateral half of the hard palate crossing the midline
 |
and firm in consistency. Right side submandibular lymph node was palpable and mobile. Clinical differential diagnosis included a carcinoma palate, malignant neoplasm of minor salivary glands. Computed Tomography showed soft tissue enhancing mass approximately 5 X 4 c.m in dimension crossing the midline of palate, extending into nasal floor and orbital floor. (Figure 3 & 4)
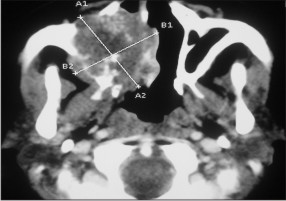 | Fig. 3-: Showing radiolucent mass crossing the midline approximately 5 x 6 c.m in dimension.
 |
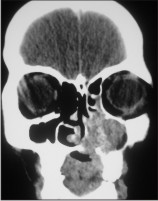 | Fig. 4-: Showing radiolucent mass extends into nasal floor and in the orbital floor.
 |
Incisional biopsy was performed for the histopathological diagnosis. The hematoxylin and eosin stained sections showed lesional tissue composed of ductal epithelial cells arranged in a cribriform pattern and in strands and cords. (Figure 5) Mixed population of cells were seen with the central area showing cells with angulated, hyperchromatic nuclei (myoepithelial cells) and peripheral cells with dysplasia. (Figure 6) Centrally located cells showed secretion resembling eosinophilic coagulum. The histopathological impression was that of polymorphous low grade adenocarcinoma.
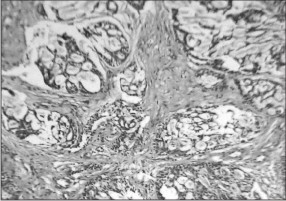 | Fig. 5-: Section showing cribriform arrangement of proliferating ductal epithelial cells with mucoid secretion (100x).
 |
 | Fig. 6-: Section showing tubular arrangement of ductal cells, mixed population of cells was seen with the central area showing cells with angulated, hyperchromatic nuclei that is myoepithelial cells (100x).
 |
Discussion
PLGA is a malignant epithelial tumor characterized by cytologic uniformity, morphologic diversity, an infiltrative growth pattern and low metastatic potential.(2) Earlier, this tumor was diagnosed as adenocarcinoma NOS, pleomorphic adenoma, adenoid cystic carcinoma.(3) It was recognized as distinct entity only since early 1980s. It was also called as lobular carcinoma, terminal duct carcinoma and low grade papillary adenocarcinoma.(4,5,6)
PLGA is the second most common intraoral malignant salivary gland tumor; it accounts for 26% of all carcinomas. It occurs almost exclusively in the minor salivary glands, commonest site of occurrence being the palate followed by buccal mucosa, upper lip, retromolar area and base of tongue. Uncommon locations include major salivary glands, lacrimal glands, nasopharynx and nasal cavity.(2,6,7)
The tumor commonly occurs in 5th -7th decade of life. There is a female predominance with female to male ratio of 2:1.(2) Clinically, the tumor presents as an asymptomatic slow growing painless mass and occasionally associated with bleeding or discomfort.(3) PLGA is low grade, indolent neoplasm which is locally invasive with infrequent recurrence or lymph node metastasis. The tumor is occasionally seen to invade or infiltrate the underlying bone.(4)
Histopathologically, the tumour cells are small to medium size and uniform in shape with bland, minimally hyperchromatic, oval nuclei and only occasional nucleoli.[8] Mitoses are uncommon and necrosis is not typical. The striking feature of these carcinomas is the variety of morphologic configurations between tumours and within an individual tumour. The main microscopic patterns are: 1) lobular, 2) papillary or papillary-cystic (typically focal), 3) cribriform areas sometimes resembling those in adenoid cystic carcinoma, and 4) trabecular or small, duct-like structures lined by a single layer of cuboidal cells.2 A relatively diagnostic pattern of growth in PLGA is the targetoid lesion, which is formed by cords and narrow tubules arranged in streamlined concentric whorls around blood vessels and nerves.[4] Foci of oncocytic, clear, squamous or mucous cells may be found. Stroma may show areas of mucinosis or hyalinization. Despite the innocuous cytologic appearance, the neoplasm always invades adjacent soft tissues and is uncapsulated. Perineural invasion is common in PLGA. Invasion of adjacent bone may be seen in tumours of the palate or mandible. Psamoma bodies and collagenous or tyrosine type crystalloids can be found in some cases.[2,9,10]
PLGA is best treated by wide surgical excision, sometimes including resection of the underlying bone. (3,9) The overall survival rate of patients with PLGA is excellent. A review of series with large numbers of cases and with long-term follow-up revealed a local recurrence rate between 9% and 17% and a regional metastases rate from 9-15% .(2)
ACKNOWLEDGEMENTS: None
References:
1. Rajendran R. In: Rajendran R, Sivapathasundharam B, editors. Tumors of Salivary glands. 6th ed. Shafer’s Textbook of Oral Pathology.pg. 235-236.
2. Luna M.A, Wenig B.M. In: Leon Barnes, John Eveson, Peter Reichart, David Sidransky, editors. Polymorphous low grade adenocarcinoma. World Health Organization classification of tumors: Pathology and Genetics, head and neck tumors. Lyon: IARC Press; 2005.pg. 223-224.
3. Dardick I. In: Dardick I, editor. Polymorphous low grade adenocarcinoma. 4th ed. Color atlas/text of salivary gland tumor pathology. New York: Igaku-Shoin. pg. 211-221.
4. Cheuk W, Chan J.K.C. In: Fletcher, editor. Salivary gland tumors. 2nd ed. Soft tissue tumors.pg. 275-277.
5. Neville W, Damm D, Allen C, Bouquot J. In : Salivary gland Pathology. 3rd ed. Oral and maxillofacial Pathology.pg. 497-98.
6. Aberle AM, Abrams AM, Bowe R, Melrose RJ, Handlers JP. Lobular carcinoma of minor salivary glands: A clinicopathologic study of twenty cases. Oral Surg Oral Med Oral Pathol 1985;60:387-95.
7. Regezi JA, Zarbo RJ, Stewart JC, Courtney RM: Polymorphous low-grade adenocarcinoma of minor salivary gland: A comparative histologic and immunohistochemical study. Oral Surg Oral Med Oral Pathol 1991;19:121-29.
8. Slootweg PJ: Low-grade adenocarcinoma of the oral cavity- polymorphous or papillary. Oral Pathol Med 1993;22:327-30.
9. Vincent SD, Hammond HL, Finkelstein MW: Clinical and therapeutic features of polymorphous low-grade adenocarcinoma. Oral Surg Oral Med Oral Pathol 1994;77:41-47.
10. Clayton JR, Pogrel A, Regezi JA: Simultaneous multifocal polymorphous low-grade adenocarcinoma: Report of two cases. Oral Surg Oral Med Oral Pathol 1995;80:71-77. |
|
|
|
|
|
|