Introduction
Hormones are specific regulatory molecules that have potent effects on the major determinants of the development and the integrity of the skeleton and oral cavity including periodontal tissues. It is clear that periodontal manifestations occur when an imbalance of these steroid hormones take place. The Bacterial plaque has been established as the primary etiologic factor for the initiation of periodontal disease.1 However, it has also been shown that without a susceptible host the periodontal pathogens are necessary but not sufficient for disease to occur. Sexual hormones have been suggested as important modifying factors that may influence the pathogenesis of periodontal diseases. 2, 3
Steroid Sex Hormones
Steroid sex hormones are derived from cholesterol and as a common structure they have three rings of six carbon atoms. They are believed to play an important role in the maintenance of the skeletal integrity, including the alveolar bone. The steroid sex hormones such as estrogen and estradiol have been known for their effect on bone mineral metabolism. Other bone turnover-related hormones include progesterone, testosterone and dihydrotestosterone, androstenedione, dihydroepiandrostenedione, and sex hormone-binding globulin. Among these, estrogens, progesterone, and testosterone have been most linked with periodontal pathogenesis. 3
Effects of androgens on the periodontal tissues 4
Inhibit prostaglandin secretion
Enhance osteoblast proliferation and differentiation
Reduce IL-6 production during inflammation
Enhance matrix synthesis by periodontal ligament fibroblasts and osteoblasts 4
Effects of estrogen on the periodontal tissues4
Decreases keratinization while increasing epithelial glycogen that results in the diminution in the effectiveness of the epithelial barrier
Increases cellular proliferation in blood vessels
Stimulates PMNL phagocytosis
Inhibits PMNL chemotaxis
Suppress leukocyte production from the bone marrow
Inhibits proinflammatory cytokines released by human marrow cells
Reduces T-cell mediated inflammation
Stimulates the proliferation of the gingival fibroblasts
Stimulates the synthesis and maturation of gingival connective tissues
Increases the amount of gingival inflammation with no increase of plaque. 4
Effects of progesterone on the periodontal tissues 4
Increases vascular dilatation, thus increases permeability
Increases the production of prostaglandins
Increases PMNL and prostaglandin E2 in the gingival crevicular fluid (GCF)
Reduces glucocorticoid anti-inflammatory effect
Inhibits collagen and noncollagen synthesis in periodontal ligament fibroblast
Inhibits proliferation of human gingival fibroblast proliferation
Alters rate and pattern of collagen production in gingiva resulting in reduced repair and maintenance potential
Increases the metabolic breakdown of folate which is necessary for tissue maintenance and repair. 4
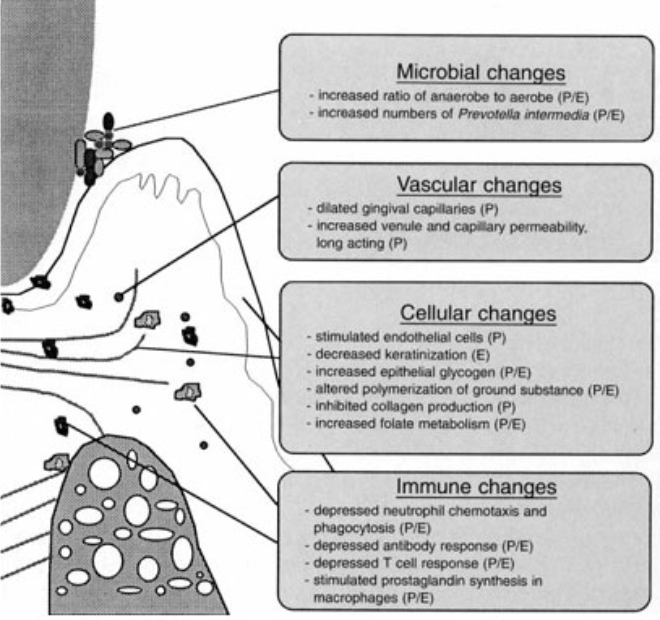 | Fig 1
 |
Influence of estrogen and progesterone on the periodontal environment. P: effect of progesterone; E: effect of estrogen. 5
Courtesy: (Amar, Chung 1994) 5
Factors Infuencing Sex Hormone Effects on the Periodontium
1) Gender
2) Age
3) Hormone supplements
1) Gender
Gender plays an important role in changes of the bone density throughout the entire skeleton. It is also known that women are much more affected than men (e.g. osteoporosis). Lau et al. (2001) reported that 80% of the osteoporotic patients are female, correlating with the higher frequency of hip fractures in females, who are also more likely to experience hormonal imbalance throughout their lives than males. 6 In addition, when the influence of gender on periodontal disease was studied, females were considered for several years to be more affected than males, although contradicting data have been reported. This disparity seems to be simply correlated with the fact that females are more likely to seek dental care than males. 3
2) Age
The biological changes on the periodontal tissues during different time points such as puberty, the menstrual cycle, pregnancy, menopause, and oral contraceptive use have heightened interest in the relationship between steroid sex hormones and the health of the periodontium. Females seem to be more prone to hormone imbalance than males.
I) Puberty
Puberty is a complex process of sexual maturation resulting in an individual capable of reproduction. 3, 7 It is also responsible for changes in physical appearance and behavior,3,8,9,10 that are related with increased levels of the steroid sex hormones, testosterone in males and estradiol in females During puberty, the production of sex hormones increases to a level that remains constant for the entire normal reproductive period. Changes in hormone levels have been related with an increased prevalence of gingivitis followed by remission 3, a situation that is not necessarily associated with an increase in the amount of dental plaque. 3,11 The sub gingival microflora is also altered during this period since the bacterial counts increase in number, and there is a prevalence of certain bacterial species such as Prevotella intermedia (Pi) and Capnocytophaga species.3,12,13 Pi has been shown to possess the ability to substitute estrogen and progesterone for menadione (vitamin K) as an essential growth factor.14 Capnocytophaga species, which often increase during puberty, have been associated with the increased bleeding tendency observed during this period of time. 3, 13
Clinical and microbial changes in the periodontal tissues during puberty
Increased gingival inflammation without accompanying an increase in plaque levels.
Increased prevalence of certain bacterial species such as P.intermedia and Capnocytophaga species. 4
ii) Menstrual cycle
The menstrual cycle is controlled by the secretion of sex hormones over a 25-30-day period and is responsible for continued ovulation until menopause. 3, 15 In humans, the menstrual cycle can be divided into two phases: a follicular or proliferative phase, and a luteal or secretory phase. During the first phase, there is an increase in estrogen levels. At the same time, the luteinizing hormone stimulates progesterone secretion and ovulation. After ovulation, the luteal phase is characterized by an increase in progesterone and estrogen secretion. At the end of this phase, and if fertilization has not occurred, the plasma levels of progesterone and estradiol decline because of the demise of the corpus luteum. Generally, the periodontium does not exhibit evident changes during the menstrual cycle. Nonetheless, two different clinical findings have been observed in the oral cavity: gingival bleeding and increased production of gingival exudate. 3, 16
Clinical changes in the periodontal tissues during menstruation
Bleeding and swollen gingiva
An increase in gingival exudate
A minor increase in tooth mobility 4
iii) Pregnancy
Some of the most remarkable endocrine alterations accompany pregnancy. During this period, both progesterone and estrogen are elevated due to continuous production of these hormones by the corpus luteum. By the end of the third trimester, progesterone and estrogen reach peak plasma levels of 100 and 6 ng/ml, respectively, which represent 10 and 30 times the levels observed during the menstrual cycle. Susceptibility to infections (e.g. periodontal infection) increases during early gestation due to alterations in the immune system 3, 17 and can be explained by the hormonal changes observed during pregnancy 18, suppression on T-cell activity, decreased neutrophil chemotaxis and phagocytosis, altered lymphocyte response and depressed antibody production 19, chronic maternal stress, and even nutritional deficiency associated with increased nutritional demand by both the mother and the fetus. These immunologic changes might also be responsible for periodontal pathologic conditions observed during pregnancy such as pregnancy gingivitis 20, 21, pregnancy granuloma, periodontitis, and dental caries. The increased synthesis of PGE2 observed when estradiol and progesterone are present in higher concentrations, such as occurs during pregnancy, may also contribute to these pathologic changes. 3 On the other hand, periodontal pathogens such as Pi and Porphyromonas gingivalis (Pg) can also use female sex hormones such as progesterone or estradiol as a source of nutrients. These bacteria are generally increased in the gingival crevicular fluid of pregnant women, a situation that is positively correlated with the severity of pregnancy gingivitis. 3
Clinical and microbial changes in the periodontal tissues during pregnancy
Increased gingival probing depths
Increased gingival inflammation
Increased gingival crevicular fluid flow
Increased bleeding upon probing
Increased tooth mobility
Increased incidences of pyogenic granulomas
Increased numbers of periodontopathogens especially P. gingivalis & P.intermedia 4
iv) Menopause and postmenopause
In the premenopausal women, the principal circulating estrogen is 17b-estradiol. As women approach menopause, the levels of estrogen begin to drop mainly during the late follicular and luteal phase of the menstrual cycle. 22 As a result of this physiologic situation, irregular cycles start to occur. Frequently, the time frame between regular cycles and the cessation of menstrual periods, called perimenopausal transition, is 2-7 years. During this period, the concentration of circulating estrogen decreases while follicle-stimulating hormone (FSH) and luteinizing hormone (LH) concentrations increase. Consequently, the effects of estrogen are reduced, therefore compromising the anti-inflammatory effect of this hormone on the periodontium. 3
Progesterone is another sex hormone that may play an important role in bone metabolism during pre- and post menopause. 23 It is believed that ovarian deficiency and associated alterations, but not aging, are the predominant causes of bone loss during the first two decades after menopause. Researches have shown that progesterone may compete with glucocorticoids for an osteoblast receptor and inhibit the glucocorticoid-induced osteoporosis. Therefore, postmenopausal bone density reduction may be the result of a combination of the inhibition of osteoclast downregulation by reduced estrogen and the increased cortisol inhibition of osteoblasts via the reduction of competition with progesterone. 3
Clinical changes in the periodontal tissues during menopause and postmenopause
Reduction in epithelial keratinization
A reduction in salivary gland flow
Drying of the oral tissues
Redness and abnormal paleness of the gingival tissues
Bleeding on probing and brushing 4
3) Hormone replacement
As addressed above, females experience hormonal changes under both physiological (e.g. menstrual cycle, pregnancy) and nonphysiological conditions (e.g. hormone therapy, use of oral contraceptives.
I) Contraceptives
The influence of contraceptives on the periodontium is increases in inflammation and in the amount of gingival exudates, increase in the prevalence of dry socket after dental extraction, and accelerated progression of periodontal disease (higher gingival index scores and more loss of attachment). 3
Impact of contraceptives on clinical and microbial features of periodontal tissues
Inflammation ranges from mild edema and erythema to severe inflammation with hemorrhagic or hyperplastic gingival tissues
A 50 per cent increase in gingival fluid volume
A 16-fold-increase in Bacteroides species 4
ii) Hormone replacement therapy in postmenopausal women
Estrogen deficiency is the dominant pathogenic factor for osteoporosis in women.24 Although hormonal replacement in an adequate dosage can slow or prevent bone loss 25, only a small percentage of postmenopausal women receive such therapy, and many who do fail to comply with the prescribed regimen because of the fear of cancer, irregular bleeding, and other minor side effects. Progesterone alone is not effective in preventing postmenopausal bone and tooth loss 26, but when combined with estrogen it is believed to uncouple formation and resorption to diminish bone resorption induced by estrogen. 3
Clinical changes in the periodontal tissues during menopause and postmenopause
Reduction in epithelial keratinization
A reduction in salivary gland flow
Drying of the oral tissues
Redness and abnormal paleness of the gingival tissues
Bleeding on probing and brushing 4
Effects of HRT on the periodontal tissues
A protection takes place against tooth loss
Reduction in gingival bleeding
Reduction in the risk of edentulousim 4
Hormonal influences on the microbiota
The effects of sex steroid hormones on the subgingival microbiota during pregnancy have been well documented. Kornman & Loesche 27 reported that during the second trimester, plaque levels remained constant, yet gingivitis and gingival bleeding were shown to increase in severity. 28 At the same time, the ratio of subgingival bacterial anaerobes-to-aerobes increased, as well as proportions of Bacteroides melaninogenicus and P. intermedia (2.2-10.1%). Subgingival plaque samples from these patients during the second trimester demonstrated a significantly higher accumulation of estradiol and progesterone than plaque samples at other time periods. Subsequently, both estradiol and progesterone were shown to be selectively accumulated by P. intermedia as a substitute for vitamin K, and thus postulated to be acting as a growth factor for this microorganism. Not all studies have corroborated these findings, and Jonsson et al. 29 found no difference in levels of P. intermedia at any time during pregnancy or between pregnant and nonpregnant controls in a cross-sectional assessment. This has led to speculation that the increase in P. intermedia seen during the second trimester of pregnancy may actually be independent of estrogens or progesterone and may occur for other reasons. Mariotti 2 has made observations in this regard. First, P. intermedia is seen to increase during the second trimester of pregnancy followed by a decline to postpartum values during the third trimester, despite highly elevated hormone levels still present during the third trimester. Additionally, there was no analysis of competitive inhibition with other steroid-like molecules performed in the heretofore cited studies; therefore, it is open to question whether the accumulation of estradiol or progesterone in second trimester plaque samples or pure cultures of P. intermedia was sex steroid hormone specific or merely dependent on the lipophilic nature of the plaque sample. 28
Hormonal influences on the gingival vasculature
The effects of estrogens and progestins on the gingival vasculature could potentially explain the increased edema, erythema, gingival crevicular exudate, and hemorrhagic gingival tissues noted during pregnancy as well as other stages of the reproductive cycle. An increase in gingival crevicular fluid flow has been correlated to elevated sex steroid levels, which indicates that these hormones may affect vascular permeability in the gingival sulcus. 28
Hormonal influences on cells of the periodontium
The effects of sex steroid hormones on individual cells of the periodontium may also play a significant role in the exaggerated gingival responses seen during the female reproductive cycle and pregnancy. Sex steroid hormones have been shown to directly and indirectly exert influence on cellular proliferation, differentiation, and growth in target tissues, including keratinocytes and fibroblasts in the gingiva.2 Two theories for the actions of the hormones on these cells involve the role hormones may play in altering the effectiveness of the epithelial barrier to bacterial insult, and in affecting collagen maintenance and repair. Estrogens stimulate epithelial proliferation and increase keratinization of the vaginal mucosa. 5 Some evidence also exists that sex hormones may have a similar effect on the oral mucosal and gingival epithelia, and a reduction in the keratinization of gingival epithelium of postmenopausal women has been shown to accompany declining plasma estrogen levels. Fibroblast proliferation and collagen maturation in gingival connective tissues may be affected by both estrogen and progesterone. By altering collagen turnover, estrogens may stimulate the proliferation of gingival fibroblasts, and the synthesis and maturation of gingival connective tissues. Sex steroid hormones have also been shown to increase the rate of folate metabolism in oral mucosa. 30 Since folate is required for tissue maintenance, increased metabolism could deplete folate stores and inhibit tissue repair. Additionally, progesterone in concentrations corresponding to the third trimester of pregnancy has been shown to lower the synthesis of glycosaminoglycans, a major constituent of the connective tissue matrix of gingiva. 28
Influence of Sex Hormones on Periodontal/Implant Wound Healing
At a molecular level, research has also shown that sex hormones have a regulatory effect on growth factors involved in the wound healing such as the keratinocyte growth factor 31, which has been known to have wound healing regulatory effect including stimulation of proliferation, migration, and morphogenesis of pluripotential cells. However, the influence of sex hormones on periodontal wound healing is still largely unknown.3
Conclusion
Sexual hormones play an important role in influencing periodontal disease progression and wound healing. These effects are different depending on the gender as well as the lifetime period analyzed. It is also clear that not all patients and their periodontium respond in the same way to similar amounts of circulating sexual hormones. In addition, the influence of sex hormones can be minimized with good plaque control as well as with hormone replacement therapies; however, the true mechanism of how these interactions actually occur remains to be determined. 3
References
1) Loe, H., Theilade, E. Jensen, B (1965). Experimental gingivitis in man. Journal of Periodontology 36, 177-187.
2) Mariotti, A. (1994) Sex steroid hormones and cell dynamics in the periodontium. Critical Reviews in Oral Biology and Medicine 5, 27-53.
3) Mascarenhas P, Gapski R, Al-Shammari K, Wang H-L: In?uence of sex hormones on the periodontium. Journal of Clinical Periodontology 2003; 30: 671-681.
4) Güncü GN, Tözüm TF, Ça?glayan F (20050. Effects of endogenous sex hormones on the periodontium. Review of literature. Australian Dental Journal; 50 :( 3):138-145.
5) Amar SN, Chung KM (1994). Influence of hormonal variation on the periodontium in women. Periodontology 2000, 6, 79-87.
6) Lau, E. M., Suriwongpaisal, P., Lee, J. K., Das De, S., Festin, M. R., Saw, S. M., Khir, A., Torralba, T., Sham, A. & Sambrook, P. (2001) Risk factors for hip fracture in Asian men and women: the Asian osteoporosis study. Journal of Bone and Mineral Research 16, 572-580.
7) Ford, J. J. & D'Occhio, M. J. (1989) Differentiation of sexual behavior in cattle, sheep and swine. Journal of Animal Science 67, 1816-1823.
8) Buchanan, C. M., Eccles, J. S. & Becker, J. B. (1992) Are adolescents the victims of raging hormones: evidence for activational effects of hormones on moods and behavior at adolescence. Psychological Bullettin 111, 62-107.
9) Angold, A. & Worthman, C. W. (1993) Puberty onset of gender differences in rates of depression: a developmental, epidemiologic and neuroendocrine perspective. Journal of Affective Disorders 29, 145-158.
10) Angold, A., Costello, E. J., Erkanli, A., Worthman, C. M. (1999) Pubertal changes in hormone levels and depression in girls. Psychological Medicine 29, 1043-1053.
11) Sutcliffe, P. (1972) A longitudinal study of gingivitis and puberty. Journal of Periodontal Research 7, 52-58.
12) Yanover, L., Ellen, R. P. (1986) A clinical and microbiologic examination of gingival disease in parapubescent females. Journal of Periodontology 57, 562-567.
13) Gusberti, F. A., Mombelli, A., Lang, N. P., Minder, C. E. (1990) Changes in subgingival microbiota during puberty. A 4-year longitudinal study. Journal of Clinical Periodontology 17, 685-692.
14) Kornman, K. S., Loesche, W. J. (1982) Effects of estradiol and progesterone on Bacteroides melaninogenicus and Bacteroides gingivalis. Infection and Immunity 35, 256-263.
15) McCartney, C. R., Gingrich, M. B., Hu, Y., Evans, W. S. & Marshall, J. C. (2002) Hypothalamic regulation of cyclic ovulation: evidence that the increase in gonadotropin-releasing hormone pulse frequency during the follicular phase re?ects the gradual loss of the restraining effects of progesterone. Journal of Clinical Endocrinology and Metabolism 87, 2194-2200.
16) Grodstein, F., Colditz, G. A. & Stampfer, M. J. (1996a) Post-menopausal hormone use and tooth loss: a prospective study. Journal of the American Dental Association 127, 370-377, quiz 392.
17) Brabin, B. J. (1985) Epidemiology of infection in pregnancy. Reviews of Infectious Diseases 7, 579-603.
18) Hansen, P. J. (1998) Regulation of uterine immune function by progesterone-lessons from the sheep. Journal Reproductive Immunology 40, 63-79.
19) Zachariasen, R. D. (1993) The effect of elevated ovarian hormones on periodontal health: oral contraceptives and pregnancy. Women Health 20, 21-30.
20) Silness, J. & Loe, H. (1964) Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontologica Scandanavia 22, 121-135.
21) Soory, M. (2000a) Hormonal factors in periodontal disease. Dentistry Update 27, 380-383.
22) Sherman, B. M. & Korenman, S. G. (1975) Hormonal characteristics of the human menstrual cycle throughout reproductive life. Journal of Clinical Investigation 55, 699-706.
23) Katz, I. A. & Epstein, S. (1993) Bone mineral metabolism at the menopause: determinants and markers. In Humoral factors in the regulation of tissue growth, ed. Piero. P. F., Vol 5, pp. 211-223. New York: Springer-Verlag.
24) Reinhardt, R. A., Payne, J. B., Maze, C. A., Patil, K. D., Gallagher, S. J. & Mattson, J. S. (1999) In?uence of estrogen and osteopenia/ osteoporosis on clinical periodontitis in postmenopausal women. Journal of Periodontology 70, 823-828.
25) Allen, I. E., Monroe, M., Connelly, J., Cintron, R. & Ross, S. D. (2000) Effect of postmenopausal hormone replacement therapy on dental outcomes: systematic review of the literature and pharmacoeconomic analysis. Management Care Interface 13, 93-99
26) Jeffcoat, M. K. (1998) Osteoporosis: a possible modifying factor in oral bone loss. Annals of Periodontology 3, 312-321
27) Kornman KS, Loesche WJ (1980). The subgingival microflora during pregnancy. Journal of Periodontal Research: 15: 111-122.
28) Mealey BL & Moritz AJ (2003). Hormonal in?uences: effects of diabetesmellitus and endogenous female sex steroid hormones on the periodontium. Periodontology 2000, 32, 59-81.
29) Jonsson R, Howland B, Bowden G (1988). Relationships between periodontal heath, salivary steroids, and Bacteroides intermedius in males, pregnant and non-pregnant women. Journal of Dental Research: 67: 1062-1069.
30) Pack ARC, Thomson ME (1980). Effects of topical and systemic folic acid supplementation on gingivitis in pregnancy. Journal of Clinical Periodontology: 7: 402-414.
31) Rubin, J. S., Bottaro, D. P., Chedid, M., Miki, T., Ron, D., Cheon, G., Taylor, W. G., Fortney, E., Sakata, H., Finch, P. W. & LaRochelle, W. J. (1995) Keratinocyte growth factor. Cell Biology International 19, 399-411. |