INTRODUCTION:
The age of tissue engineering is upon us. Mankind is advancing beyond the ability to create inanimate objects, toward the capability of replacing and regenerating our own living body tissues. The amalgamation of bioengineering and
dentistry will result in an explosion of knowledge that will enhance our understanding of craniofacial development and culminate in a new era in dentistry, enabling us to restore lost tissue function. Tissue engineering is also referred to as
‘‘regenerative dentistry,’’ because the goal of tissue engineering is to restore tissue function through the delivery of stem cells, bioactive molecules, or synthetic tissue constructs engineered in the laboratory. The patient demand for tissue engineering therapies is staggering, both in scope and cost. Each year, $400 billion is spent treating Americans suffering some type of tissue or end-stage organ failure. These data include 20,000 organ transplants, 500,000 joint replacements, and hundreds of millions of dental and oral craniofacial procedures ranging from tooth restorations to major reconstruction of facial soft and mineralized tissues. The application of regenerative dentistry in dental clinics can produce wonderful treatments to dramatically improve patients’ of life. Historically, materials and treatment options have
provided the dentist with a limited ability to replace diseased, infected, traumatized, and lost tissues. Looking to the future, advances in bioengineering research are set to unleash the potential of the human genome project and molecular biology into dental practice.
Tissue engineering has become the new frontier in dentistry. A past frontier was the introduction of amalgam restorative materials in the 1830s. By 1845, the American Society of Dental Surgeons, an early professional organization, passed a resolution condemning the use of mercury amalgam as a toxic substance, and expelled members who practiced such use. When used properly, however, the material was longlasting and relatively easy to manipulate. Eventually, in the late 1890s, largely through work of Dr. G.V. Black, the‘‘Father of Modern Dentistry,’’the formulation and proper application
of mercury amalgam became better standardized and more successful. The use of dental amalgam has always proven to be controversial and divisive among the general public and dental profession, as it still is today. If we use dental amalgam as a
lesson on the controversy of introducing an entirely new type of dental material and treatment, it is easy to speculate that use of tissue engineering in regenerative dentistry will always prove to be controversial. Controversy surrounding
regenerative dentistry is not a bad thing, because it increases scrutiny of its safety, and helps educate the public and profession on its effectiveness and potential disadvantages.
Currently, strategies employed to engineer tissue can be categorized into three major classes: conductive, inductive, and cell transplantation approaches. These approaches all typically utilize a material component, although with different
goals.
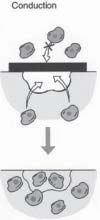
Conductive approaches (Figure 1) utilize biomaterials in a passive manner to facilitate the growth or regenerative capacity of existing tissue. An example of this that is very familiar to dentists, and particularly periodontists, is the use of barrier membranes in guided tissue regeneration. Nyman et al. were the first to successfully use Osseoconductive mechanisms in providing a means for selective wound healing by supportingthe in growth of the periodontal supporting cells,
 |
 |
FIGURE 1 - The conductive approach makes the use of a barrier membrane to exclude connective tissue cells that will interfere with the regenerative process, while enabling the desired host cells to populate the regeneration site. while excluding gingival epithelial and connective tissue cells from reconstruction sites. Techniques and materials are still being optimized in guided tissue regeneration. However, the appropriate use of barrier membranes promotes redictable bone repair and histologically verifiable new attachment with new formation of cementum and periodontal
ligament fibers.
Treatment options in restorative and prosthetic dentistry have been revolutionized by another relatively widespread application of a conductive approach, osseointegration of the dental implant. Branemark et al. were the first to successfully achieve this phenomenon, and its application is relatively simple in that the armamentarium does
not include living cells or diffusible biological signals.
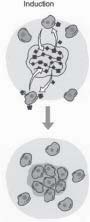
The second major tissue engineering strategy “Induction” (Figure 2) involves activating cells in close proximity to the defect site with specific biological signals. The origins of this mechanism are rooted in the discovery of bone orphogenetic proteins (BMPs). “Urist” first showed that new bone could be formed at nonmineralizing, or ectopic, sites after implantation of powdered bone (bone demineralized and ground into fine
 |
 |
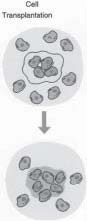
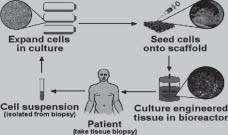
FIGURE 2 - The inductive approach uses a biodegradable polymer scaffold as a vehicle to deliver growth factors and genes to the host site. The growth factors or genes can be released at a controlled rate based on the breakdown of the polymer. particles). Contained within the powdered bone were proteins (BMPs), which turned out to be the key elements for inducing bone formation. These proteins are now available in recombinant forms and produced on a large scale by biotechnology companies. BMPs have been used in many clinical trials and are very promising as a means of therapy and supplementation in the regeneration and repair of bone in a variety of situations, including non-healing fractures and periodontal disease. One limitation of inductive approaches is that the inductive factors for a particular tissue may not known. In this situation the third tissue engineering approach, cell transplantation, becomes very attractive. This approach involves direct Transplantation of Cells (Figure3) grown in the laboratory. The cell transplantation strategy truly reflects the multidisciplinary nature of tissue engineering, as it requires the clinician or surgeon, the bioengineer, and the cell biologist. The clinician is required to biopsy a small sample of tissue containing the cells of interest. Principles of cell biology are required to multiply cells million-folds in the laboratory and maintain their function. Meanwhile, the bioengineer manufactures the tissue, in bioreactors, and the material onto which the cells will be placed for ransplantation. Lastly,
 |
 |
FIGURE 3 - The cell transplantation strategy uses a similar vehicle for delivery in order to transplant cells and partial
tissues to the host site.the clinician is required to transplant the engineered tissue. After transplantation, the polymer scaffold degrades and/or is remodeled by host and transplanted cells, resulting in a completely natural tissue. A common feature to all three of the tissue engineering strategies is that they typically employ the use of polymeric aterials. In conductive approaches, the polymer is used primarily as a barrier membrane for the exclusion of specific
cells that may disturb the regenerative process. Inductive approaches typically employ a carrier or vehicle for the delivery of proteins (e.g., BMP) or the actual DNA (gene) that encodes the protein. These molecules then directly (proteins) or
indirectly (DNA to mRNA to protein) exert their effects on cells at the anatomic site by promoting the formation of the desired tissue type. Biodegradable polymer carriers allow a localized and sustained release of the inductive molecules.
The rate and dose of molecule delivery are controlled by features (e.g., degradation rate) of the carrier. Delivery vehicles are also frequently used in cell transplantation approaches. However, in this approach the vehicle serves as a carrier of
whole cells and even partial tissues. In addition to serving as simple vehicles for delivery of cells, the vehicles also serve as scaffolds to guide new tissue growth in a predictable manner from both the transplanted cells and interacting host cells. The two major types of polymeric materials used in all three tissue engineering strategies are collagen derived from animal sources and synthetic polymers of lactic and glycolic acid (same polymer used in resorbable sutures).
Collagen is degraded by the cells in the tissue as
 |
 |
FIGURE 4 - Multidisciplinary nature of tissue engineering it develops, while the synthetic polymers degrade
into the natural metabolites lactic acid and glycolic acid by the action of water at the implant site. A variety of new materials are also being developed for these applications, and injectable materials that allow a minimally invasive delivery of inductive molecules or cells are especially attractive [3].
Practice Implications : Tissue engineering will have a considerable effect on dental practice during the next 25 years. The greatest effects will likely be related to the repair and replacement of mineralized tissues, the promotion of oral wound healing and the use of gene transfer adjunctively.
Recently, there has been a substantial and growing public1 and scientific awareness of a relatively new field of applied biological research called tissue engineering. This field builds on the interface between materials science and biocompatibility, and integrates cells, natural or synthetic scaffolds, and specific signals to create new tissues. This field is increasingly being viewed as having enormous clinical potential.
Historically, some of the earliest attempts at tissue replacement, dating back thousands of years, involved teeth. In modern times, dentistry has continued to place considerable emphasis on, and be a leader in, the study and use of
biocompatible materials. The purpose of this brief review is to provide the practicing dentist with – A general perspective and background on tissue engineering;
– A sense of what has been accomplished in this field thus far;
– A consideration of the likely impact of tissue engineering on the practice of dentistry during the next 25 years.
For a more in-depth review of this field, we recommend several articles that make up a special report, as well as recent texts on the subject.
TISSUES TO BE ENGINEERED UPON
Two important questions relevant to the dental practitioner are “What kind of impact will tissue engineering have on dentistry?” and “What oral tissues do we have the potential to engineer?” The answer to the first is still being formulated, but tissue engineering will likely have a revolutionary effect on dentistry. The answer to the second question is almost all tissue types. The effect that tissue engineering may have in the field of dentistry stems from its widespread application
to many different types of tissues related to the oral cavity, including bone, cartilage, skin and oral mucosa, dentin and dental pulp, and salivary glands.
BONE
Tissue engineering will likely have its most significant impact in dentistry via bone tissue engineering and regeneration. Bony defects secondary to injury, disease, and congenital disorders represent a major health problem. Current trategies aimed at replacing bony defects include the utilization of autografts, allografts, and synthetic biomaterials. Despite the fact that these substitutes restore stability and function to a reasonably sufficient degree, they still contain limitations. This has led to interest in engineering bone, which can be achieved using all three tissue engineering strategies. Both conductive and inductive approaches can be used to regenerate small bony defects. Guided tissue regeneration
(GTR) after periodontal surgery represents a conductive approach to regeneration of bone. BMPs, related proteins, and the genes encoding these proteins allow one to engineer bone using inductive approaches in situations where GTR is
not sufficient. In contrast, cell transplantation approaches offer the possibility of pre-forming large bone structures (e.g., complete mandible) that may not be achievable using the other two strategies. These structures may even be completely developed in the lab prior to use in large-scale reconstructive procedures.
CARTILAGE
As it relates to craniofacial reconstruction, the design of polymer scaffolds with defined mechanical and degradative properties has opened a new door to cartilage reconstruction. Cartilage destruction is associated with trauma and a number of diseases including degenerative articular cartilage destruction at the temporomandibular joint. The limited capacity of cartilaginous tissue to regenerate and the lack of inductive molecules have focused interest among researchers and manufacturers in developing cell transplantation approaches to engineer cartilage. Transplantation of cells without a carrier is now used clinically to repair small articular cartilaginous defects. 14 Investigators have also demonstrated in animal models that new cartilaginous tissue with precisely defined sizes and shapes relevant to maxillofacial reconstruction (e.g., nasal septum, temporomandibular joint) can be engineered using appropriate biodegradable scaffolds for transplanting the cells.
SKIN AND ORAL MUCOSA
The most successful application of tissue engineering to date is the development of skin equivalents. Skin tissue is needed in adjunctive esthetic treatment of individuals who are severely disfigured following severe burns, in radical
resective surgery to treat invasive cancers, and or major trauma wounds (like shotgun wounds and knife lacerations). Skin with both dermal and epidermal components is grown in the lab using a combination of cells and various polymer
carriers, and engineered skin products were the first tissue-engineered products the FDA approved for clinical use. A similar approach has also been developed for the replacement of oral mucosa, although this procedure has not yet been marketed. The engineering and transplantation of oral mucosa and gingiva could be potentially important as a new technique in periodontal graft surgery and in the treatment of gingival recession.
DENTIN AND DENTAL PULP
The production of dentin and dental pulp has also been achieved in animal and laboratory studies using tissue engineering strategies. The greatest potential for these engineered tissues is in the treatment of tooth decay. Dental caries remains one of the most prevalent young adult and childhood diseases, while the phrase “root canal” is probably the most dreaded term in dentistry. There are several ways in which one can potentially engineer lost dentin and dental
pulp. There is now evidence suggesting that even if the odontoblasts (cells that produce dentin) are lost due to caries, it may be possible to induce formation of new cells from pulp tissue using certain BMPs. These new odontoblasts can
synthesize new dentin. Tissue engineering of dental pulp itself may also be possible using cultured fibroblasts and synthetic polymer matrices. Further development and successful application of these strategies to regenerate dentin
and dental pulp could one day revolutionize the treatment of our most common oral health problem, cavities.
SALIVARY GLANDS
The most challenging goal of tissue engineering is replacement of complete organs, and significant progress has been made in efforts to engineer salivary gland function. The loss of salivary gland tissue and/or function, whether it be a sequalae to radiation therapy to treat cancer or part of a disease such Sjogren’s syndrome, is a problem that can significantly affect quality of life, particularly for medically compromised individuals. One method in treating salivary gland
functional deficiencies makes use of an inductive gene therapy approach. The aim in this approach is to make existing non-secretory ductal epithelial cells (following irradiation therapy) into secretory cells capable of fluid movement. Success in animal models has been demonstrated. Another method to restore salivary gland function employs cell
transplantation. Baum et al. have recently initiated the development of an artificial salivary gland substitute composed of polymer tube lined by epithelial cells. This relatively simple device could engraft into the buccal mucosa of patients whose salivary gland tissue has lost function, or been destroyed, and would have the physiological capacity to deliver an aqueous fluid to the mouth via the buccal mucosa. These new approaches could be very effective for treating conditions
associated with lost salivary gland function, including dysphagia, dysgeusia, rampant caries, and mucosal infections.
Tissue engineering of teeth requires the coordinated formation of correctly shaped crowns, roots, and periodontal ligament. Previous studies have shown that the dental mesenchyme controls crown morphogenesis and epithelial histogenesis during tooth development in vivo, but little is known about the inductive potential of dissociated mesenchymal cells used in ex vivo cultures. A 2- step method is described in which, by using different types of reassociations between epithelial and mesenchymal tissues and/or cells from mouse embryos, reassociations were cultured in vitro before in vivo implantation. In vitro, the reassociated tissues developed and resulted in tooth-like structures that exhibited normal epithelial histogenesis and allowed the functional differentiation of odontoblasts and ameloblasts. After implantation, the reassociations formed roots and periodontal ligament, the latter connected to
developing bone. The shape of the crown, initially suspected to depend on the integrity of the mesenchyme, could be modulated by adjusting the number of dissociated mesenchymal cells reassociated with the epithelial compartment.
Based on these results, we propose a refined strategy for tooth tissue engineering that may help to eventually generate morphologically defined teeth [5].
FUTURE DIRECTIONS/CONSIDERATIONS
The promise of tissue engineering in dentistry is great, but there exist major challenges that must be met in the next fifteen to twenty years for this new field to reach its potential application. Some of the main challenges lie not on the scientific side, but in the application of the technology. Once we fully understand how we can re-create functional, viable new tissues in the laboratory, how will we then be able to translate this knowledge to the patient population at large? A
major issue will be the cost of these therapies. Will industry be able to produce tissue products in a cost-efficient manner so the patient can afford this type of treatment? Secondly, in order for the new technology to reach the general masses, there will need to be health care centers and institutes capable of applying these engineered products. Individuals sufficiently trained to utilize these therapies will clearly be required, necessitating new training programs for these scientists, clinicians, and support teams. Another major challenge lies in the ethical concerns regarding engineering tissues. Relevant ethical issues include the source of cells (patient’s own vs. donated cells) and type (adult-donor vs. fetal cells). In addition, on what basis will it be decided who receives these new tissue therapies (according to need, ability to pay, etc.)? It is also unclear how third-party groups will react to the new technology and what they will cover. Needless to say, many different perspectives on these questions exist, based on individual, cultural, and scientific principles. This is undoubtedly an exciting time in dentistry and the biomedical community at large. In twenty to twenty-five years, dentistry as we know it today will be remarkably different, as it is now different from the way it was twentyfive years ago. Many dental schools and postgraduate programs are currently evaluating curriculum content in light of the public’s oral health care needs and in light of the many advances in genetics, cell and molecular biology,
and the materials sciences. At the predoctoral level, tissue engineering provides an ideal opportunity to incorporate a multidisciplinary learning experience into the curriculum which integrates concepts in cell biology, molecular biology, bioengineering, and biomaterials with clinical techniques in oral surgery, periodontics, restorative dentistry, and oral medicine. Students can see first-hand the interplay between the science underlying tissue engineering and the clinical application to oral disease. Such an experience would also allow students to see collaboration among biomedical scientists, dentists, and physicians, which is extremely rare in most dental school programs. At the postgraduate level, there is need to provide the community with a cadre of D.D.S./Ph.D.- trained practitioners, researchers, and educators with expertise in tissue engineering. For the practitioner, continuing education programs can increase awareness of tissue engineering as a therapeutic option for various oral health problems. These programs can also help establish
linkages between dentists in the community and tissue engineering specialists at academic health centers. Once the general public is aware of newer and better treatments, they will not accept anything less. The well-informed clinician capable of incorporating this technology into his or her practice will continue to thrive in the future.
WHAT ARE THE SUPPOSIDELY SAFTY MEASURES TAKEN IN TISSUE ENGINEERING/GENE THERAPY-A VERY IMPORTANT QUESTION FOR A RESEARCH
There is unanimity among experts that gene therapy trials should only be carried out under certain safety rules. The nature and scope of these rules and their legislative basis are, however, matters of controversy. As far as the legal
framework is concerned, one side argues that safety is adequately ensured through the network of existing regulations. The other side criticizes the current situation as a tangle of legal regulations and expresses grave doubts that this takes
adequate account of the specific hazards of gene therapy techniques. An overview of the international regulatory
mechanisms shows clearly that, despite widely varying legislative approaches, the emphasis in (legislative) efforts everywhere is on patient safety and biological safety. For example:
• There are strict test criteria for pharmaceuticals (which also apply to gene therapy), and this is one way of limiting the risks associated with gene therapy. Thus, licensing of gene therapy projects is subject to demanding requirements.
• There are ethics commissions present in all the countries; these commissions serve to ensure the maximum possible safety for the patient. In all the important countries (except Italy), the opinion (at least ‘consultative’) of the ethics commissions has to be obtained before approval is granted for conduct of gene therapy trials in humans.
· Another important safeguard is the professional ethical regulations covering the clinical applications of gene therapy. In the overwhelming majority of regulatory systems these are concerned (inter alia) with:
• Adequate clinical pre-trials
• Risk-benefit reviews in the use of gene therapy techniques on humans
• Prior patient education and consent
• Consultation with an ethical commission
• In addition to the specific statutory regulations there are also the general statutes on civil and criminal liability, which apply on a subsidiary basis.
Biological safety is ensured through various forms of legislation. All countries have a national (official) licensing authority. These are also the basis for establishing a common European licensing authority responsible for biological safety in member states.
Besides the common features indicated above, there are also differences in the ethics commissions of various countries, for example, in terms of the statutory basis of the ethics commissions, the commission’s responsibilities, and the binding nature of their votes.
• Under French law, there is a separate act (the ‘Loi Huriet’) covering the duties and responsibilities of the ethics commissions. In German law, the ethics commission’s powers are covered by section 40-I of the Drugs Act and, in Austria, by sections 30 et seq. of the Genetic Engineering Act. In Italy, on the other hand, there is no special regulation covering the responsibilities of the ethics commissions.
• In the USA, the responsibilities of the local ethics commissions are limited to projects promoted by the National Institutes of Health. The licensing procedure in the UK operates at two levels: Besides local ethics commissions, the central ethics commissions must also give its approval for every gene therapy project. With respect to the binding nature of their votes, some national ethics commissions have a purely advisory status, as for example in France. In other countries (e.g., USA, Austria, UK, and Denmark) the commission’s vote is more important and can result in refusal of approval [2].
CONCLUSION
Clearly, the future for regenerative and tissueengineering applications to dentistry is one with immense potential, capable of bringing quantum advances in treatment for our patients. The need for high-quality research in the basic sciences is paramount to ensuring that the development of novel clinical treatment modalities is underpinned by robust mechanistic data, and that such approaches are effective. This translational model epitomizes how dentistry should volve and highlights the need for close partnerships between basic and clinical scientists. Advances in tissue
engineering provide an increased level of understanding of the mechanical and chemical stimuli that regulate tissue responses. Oral tissue engineering can be applied to recreate missing osseous or dental structures or correct orofacial
deformities, changing the patient’s smile, midfacial height, and the soft tissue drape. Biomechanical principles can also be applied to tissue engineering to enhance the bone/tooth or bone/implant functionality and long-term stability.
Advancements are also being achieved in the area of biomimetics that will allow the creation of new biologic replacements for missing oral structures. The opportunity for bioengineering to charter the course of tooth regeneration is an exciting prospect and will improve the quality of life for patients for decades to come[1]. The benefits of regenerative endodontics include the ability to continue root development in immature teeth and to revitalize diseased teeth, which may restore their ability to heal in response to disease and trauma. The ideal design of the dental pulp constructs is to be the same shape as gutta-percha cones to accomplish a good fit when inserted into the root canal. The ideal design of the periodontal constructs is to be the same shape as general periodontal barrier membranes, and its benefits
include the replacement of diseased and traumatized periodontal tissues. This is an exciting time for biomedical science and its application. Clinical dental practice in 2025 will certainly be different.
REFERENCES
1. Earthman J.C, Sheets C.G,et al. Tissue engineering in dentistry; Clinical Plast Surg.2003 Oct:30(4):621-639
2. Parimala Tyagi, Manpreet Kaur Dhindsa. Tissue engineering and its implications in dentistry; INDIAN J DENT
RES,20(2),2009
3. Darnell Kaigler, David Mooney.Tissue Engineering’s Impact on Dentistry J OF DENT EDUCATION,65(5)2001
4. BRUCE J. BAUM and DAVID J. MOONEY. The impact of tissue engineering on dentistry ;JADA 2000: 131(3),309-318
5. Bing Hu, Amal Nadiri, Sabine Kuchler-Bopp, Fabienne Perrin-Schmitt, Heiko Peters, Hervé Lesot. Tissue Engineering of Tooth Crown, Root, and Periodontium ; J of periodontol 2000 August 2006, 12(8): 2069-2075
6. Schallhorn RG. The use of autogenous hip marrow biopsy implants for bony crater defects. J Periodontol 1968;39:145–7
7. F. BERTHIAUME and M. L. YARMUSH, in “The Biomedical Engineering Handbook,” edited by J. D. Bronzino (CRC Press LLC, Boca Raton, Florida, 2000).
8. L. L. HENCH and J. WILSON (eds.), “Introduction to Bioceramics” (Singapore World Scientific, Singapore, 1993).
9. R. LI, A. E. CLARK and L. L. HENCH, in “Chemical Processing of Advanced Materials,” edited by L. L. Hench
and J. K. West (Wiley, New York, 1992) p. 627.
10. L.L. HENCH and J. WILSON (eds.), “Clinical Performance of Skeletal Prostheses” (Chapman and Hall, London, 1996). |